Patient Referrals: A Game-Changer for Dermatology Clinics
Referral MD
DECEMBER 8, 2023
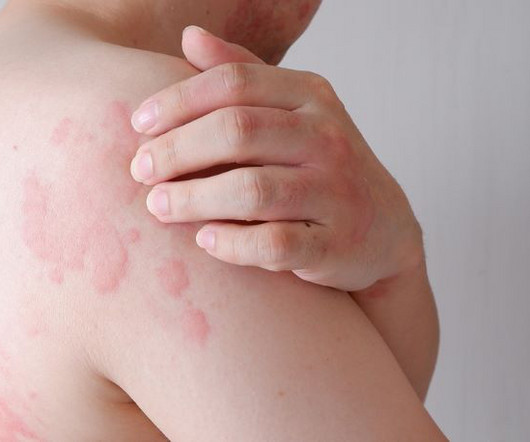
Discover the best practices to enhance, improve, and boost patient referrals for your dermatology clinic. Understanding the Importance of Patient Referrals Patient referrals play a crucial role in the success of dermatology clinics. When patients refer someone to your clinic, they are satisfied with your services.







































Let's personalize your content