After swell of biopharma outrage, Supreme Court takes up high-profile mifepristone case
Fierce Pharma
DECEMBER 13, 2023
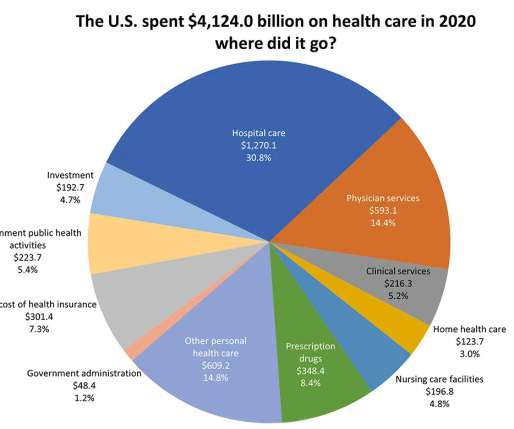
That's because the original verdict questioned the FDA's decision-making authority, prompting amicus brief submissions from high-profile industry players. . | The Supreme Court's final decision could have consequences beyond abortion access.



































Let's personalize your content