Handling HPAPIs safely – what does it take?
European Pharmaceutical Review
MARCH 9, 2023
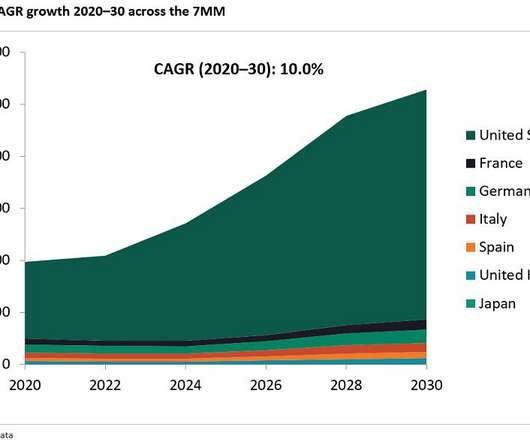
There are emerging trends in oncology, with new chemical entities (NCEs) and antibody-drug conjugates (ADC) leading to increased demand for HPAPI manufacturing capabilities. Based on the latest report and data, HPAPI market size is expected to reach $32 billion by 2028. What training is required for employees to handle HPAPIs safely?
















Let's personalize your content