UK agency pilots biobank to study links between genetics and drug side effects
Pharmaceutical Technology
MAY 25, 2023
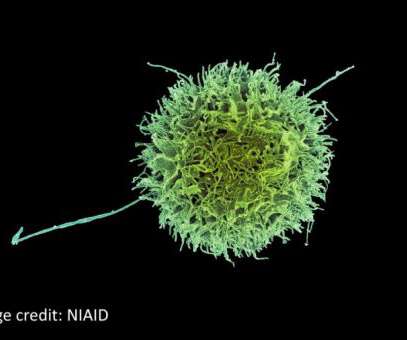
The biobank will work in parallel with the MHRA’s Yellow Card website, which is used for reporting adverse events and side effects caused by medicines and medical devices, per the 25 May announcement. Side effects due to drugs are responsible for one in every 16 hospital admissions in the UK, based on the announcement.


























Let's personalize your content