UK regulators warn of ocular side effects after treatment with Sanofi's Dupixent
Fierce Pharma
NOVEMBER 30, 2022
UK regulators warn of ocular side effects after treatment with Sanofi's Dupixent. Wed, 11/30/2022 - 10:55.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

Fierce Pharma
NOVEMBER 30, 2022
UK regulators warn of ocular side effects after treatment with Sanofi's Dupixent. Wed, 11/30/2022 - 10:55.

Teachntest
SEPTEMBER 8, 2022
Advertisement Dipane Tablets Uses, Benefits & Side Effects Dipane Tablet is a pain-relieving medicine containing Diclofenac and Paracetamol. The Dipane Tablet must … Dipane Tablets-Uses, Benefits & Side Effects (2022) Read More »
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Teachntest
SEPTEMBER 12, 2022
It’s best to take an O2 Tablet … O2 Tablets- Uses, Benefits and Side Effects (2022) Read More » It works well to treat a wide variety of bacterial infections, including those in the teeth, lungs, digestive tract, urinary tract, and genital tract.
European Pharmaceutical Review
JANUARY 4, 2024
Immunotherapy drugs like teclistamab can result in potentially fatal side effects, including CRS and immune cell-associated neurotoxicity syndrome (ICANS). In 2022, the drug was approved by the US Food and Drug Administration (FDA) for these patients. We are going with full steam into an era of immunotherapy.”

Teachntest
SEPTEMBER 23, 2022
Bifilac, Pre & probiotics will help to … Bifilac Tablet Uses, Benefits and Side Effects (2022) Read More »

World of DTC Marketing
JANUARY 1, 2022
1ne: The value proposition of prescription drugs still outweighs potential side effects – Wegovy’s demand quickly exceeded supply after the FDA approved once-weekly semaglutide injections. The post A realistic view of healthcare in 2022. Here are things I believe are more realistic.

European Pharmaceutical Review
NOVEMBER 14, 2022
The CHMP endorsed measures recommended by the EMA Pharmacovigilance Risk Assessment Committee (PRAC), to minimise the risk of serious side effects (cardiovascular conditions, blood clots, cancer and serious infections) of janus kinase (JAK) inhibitors for treating several chronic inflammatory disorders. 5 in children five to 11 years.
Pharmaceutical Technology
JANUARY 6, 2023
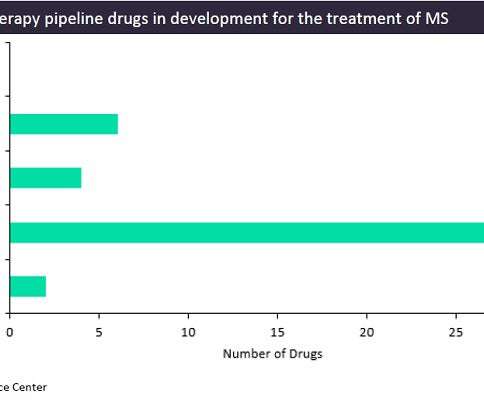
While the treatment options for multiple sclerosis (MS) patients are growing each year with the approval of new agents, all of the currently marketed treatments only slow the disease’s progression and sometimes carry risks of severe side effects, such as liver failure or the development of viral infections.
Clarivate
JANUARY 3, 2023
The latest annual edition, Research Fronts 2022, marks the 9th collaboration between Clarivate and the CAS. Moving beyond early descriptions of the SARS-CoV-2 virus and its clinical course, the 2022 Research Fronts chronicle the ongoing search for effective vaccines and treatments. Read the new report now. Fields and nations.

Medico Reach
AUGUST 25, 2022
This supply soon slowed down during the first quarter of 2022. Biotech Startup Funding Slows Down Again in 2022. The record-breaking year for Biotech funding opened up to a much cooler market in 2022. Where June 2021 saw nearly 45 Biotech startups going public, June 2022 stared at an almost stagnant number of 14.

European Pharmaceutical Review
OCTOBER 31, 2022
The Pharmacovigilance Risk Assessment Committee (PRAC) , the European Medicines Agency (EMA)’s safety board, has confirmed it recommends the withdrawal of Marketing Authorisation Application (MAA) for amfepramone obesity medicines, to prevent more patients being at risk of serious side effects. Risks of amfepramones.

PM360
AUGUST 9, 2022
Accordingly, multiple reasons for subpar patient adherence are gaining attention, including fear of side effects, lack of medical understanding, or even financial limitations. Today, with the industry’s patient-centric approach, we see more reliance on behavioral science to gain a holistic understanding of the patient journey.

PM360
SEPTEMBER 15, 2022
To guide fast service improvements, it’s particularly relevant to measure satisfaction; distress; convenience; emotional reactions of daily care; and barriers, hassles, and challenges related to management and side effects.

PM360
OCTOBER 24, 2022
With all of this, GEMTESA was shown to be safe and tolerable—with only 2% of study participants discontinuing GEMTESA due to side effects. The most common side effects seen were headache, urinary tract infection, nasal congestion, sore throat or runny nose, diarrhea, nausea, and upper respiratory tract infection.
Medgadget
AUGUST 29, 2022
Used as a treatment for bipolar disorder and depression, lithium requires very accurate and sensitive dosing, with too little providing no therapeutic benefit but slightly too much potentially leading to unwanted side-effects. Another issue with the drug is the potential for poor patient compliance. Via: ACS.

European Pharmaceutical Review
NOVEMBER 2, 2023
This evidence included comparisons of the products on an analytical level using chemical and biological tests and assays that confirmed similarity in the structural and functional features of Wezlana and Stelara, as well as comparative human pharmacokinetic data, clinical immunogenicity data and other clinical safety and effectiveness data.

Pharmaceutical Technology
JUNE 12, 2023
The two companies announced that the Clarity AD trial met both the primary and all key secondary endpoints in September 2022. However, side effects known as amyloid-related imaging abnormalities, which often involve brain swelling, were also reported, and consequently the treatment has a warning on this specific side effect on its label.

World of DTC Marketing
NOVEMBER 5, 2021
Novo Nordisk has admitted that it would not be able to keep up, adding that it would likely take until early 2022 for the supply to stabilize and that some patients “are experiencing an approximate one month or longer delay in filling prescriptions for Wegovy.” Many factors contribute to obesity.

Referral MD
JULY 28, 2022
In this piece, we will be looking at a few ways healthcare organizations- both small and big- can think about growing their practice in 2022. As the uncertainties continue to persist in 2021, the only businesses that will survive and emerge out victorious are the ones that look at new ways to pivot and quickly adapt to their surroundings.

pharmaphorum
DECEMBER 4, 2022
— Neuralink (@neuralink) December 2, 2022. — Physicians Committee (@PCRM) November 30, 2022. Thank you for joining our live stream to hear about the progress the team has made in the last year. In case you missed it, here’s the full recording: [link]. Take action! Retweet this thread and tell @elonmusk to release the tapes!
European Pharmaceutical Review
SEPTEMBER 26, 2022
At the 2022 European Society for Medical Oncology Congress (ESMO) conference, promising results from a Phase I trial, suggesting that a novel genetically modified (GM) herpes virus eradicated or reduced advanced cancers, were presented. Side effects of being injected with RP2 were mild. No serious side effects were reported.

European Pharmaceutical Review
JUNE 1, 2023
These can have significant side-effects and can be ineffective for some people. The small molecule CGRP antagonist was approved in April 2022, granting it the first marketing authorisation by the European Commission (EC) for both the treatment and prevention of migraines. These are all administered as injections.

pharmaphorum
SEPTEMBER 16, 2022
Findings presented at EADVC 2022. Minor side effects to long-term maintenance balance. Any clinical study seeks to achieve maximum results efficacy with minimal occurrence of side effects. However, side effects do happen.

European Pharmaceutical Review
JANUARY 30, 2023
The organisation issued a revised opinion to minimise the risk of serious side effects with Janus kinase (JAK) inhibitors used to treat several chronic inflammatory disorders. These side effects include cardiovascular conditions, blood clots, cancer and serious infections.

PM360
SEPTEMBER 28, 2022
Nearly half of oncologists, including hematology-oncologists and medical oncologists, allude to treatment challenges including side effects and patient comorbidities, and seek out medical information to address these challenges. 2022 , which was conducted in the spring and summer of 2022. In contrast, half of U.S.
European Pharmaceutical Review
MAY 23, 2024
CAR T-cell Therapy and its side effects [Internet]. References National Cancer Institute. CAR T Cells: Engineering Immune Cells to Treat Cancer [Internet]. National Cancer Institute. Cancer.gov. Available from: [link] American Cancer Society. www.cancer.org.
World of DTC Marketing
AUGUST 1, 2022
They can be expensive and have serious side effects. And those trends have only accelerated into 2022. . “New weight loss drugs help patients lose significant weight,” but very few are telling the whole story. However, there are essential things to know about all of these medications.

Pharmaceutical Technology
NOVEMBER 14, 2022
Such developments, including a 2021 approval of AstraZeneca’s Saphnelo (anifrolumab) , prompted The Lancet Rheumatology to posit that 2022 could be a “banner year” for the condition. In the five-patient report, mild cytokine release syndrome, a common side effect with CAR-Ts was observed, but the treatment was considered well tolerated.

PM360
MAY 25, 2023
above pre-pandemic levels by Q2 2022. They’re wonderful medications, blockbuster sales, but real side effects. Another side effect that tends to be related to weight gain is the metabolic syndrome, which increases your risk for cardiac events and that increases your risk for sudden death and heart attacks.

Nixon Gwilt Law
NOVEMBER 5, 2021
On November 2, 2021, the Centers for Medicare and Medicaid Services (“CMS”) finalized the Medicare Physician Fee Schedule for Calendar Year 2022 (the “Final 2022 MPFS” or the “Final Rule”). This means that, even if the PHE ends in 2022, providers may bill for Category 3 telehealth services until the close of the following year.
Pharmaceutical Technology
JANUARY 27, 2023
The FDA’s recent approval of TG Therapeutics’s Briumvi (ublituximab) against relapsing forms of multiple sclerosis (MS) is welcome news for the company after suffering a major setback in 2022 when the FDA extended its review of Briumvi by three months. Some 48% of patients on Briumvi also suffered with infusion reactions.

European Pharmaceutical Review
SEPTEMBER 20, 2022
This interchangeability refers to the fact that a reference medicine can be substituted by a biosimilar, without risk, side effects or outcomes different to the original drug. The Committee for Medicinal Products for Human Use (CHMP) endorsed the statement on 22 July 2022.
European Pharmaceutical Review
AUGUST 16, 2022
Safety monitoring showed that the side effects observed were the same as those seen for the original Moderna booster dose and were typically mild and self-resolving. In an exploratory analysis the bivalent vaccine was also found to generate a good immune response against the Omicron sub-variants BA.4

pharmaphorum
AUGUST 4, 2022
Currently, ANCA vasculitis is treated with rituximab combined with corticosteroids, which can have serious side effects when administered over prolonged periods. Amgen said it expects the deal to close in the fourth quarter of 2022. The post Amgen splashes out on ChemoCentryx and its blockbuster hopeful appeared first on.

World of DTC Marketing
JUNE 21, 2021
SKIMMERS SUMMARY: Google has vowed to block cookies completely on its Chrome browser, which is used by around 70 percent of the world’s desktop computer owners, by the beginning of 2022. Display ads are usually reported to have an overall click rate of about five clicks in ten thousand ads served.

pharmaphorum
DECEMBER 7, 2022
Neuralink has already come under the scrutiny of groups, including the Physician’s Committee for Responsible Medicine, which has called for the company to release data from its animal experiments, including side effects from the implant procedure, such as infections. — Physicians Committee (@PCRM) November 30, 2022.
Pharmaceutical Technology
JANUARY 30, 2023
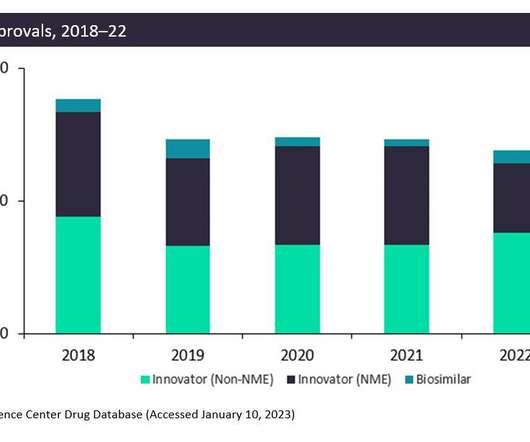
The FDA approved fewer innovative drugs, New Molecular Entities (NMEs), in 2022 than it did in 2021: only 42 drugs compared to 59 drugs. However, non-NME and biosimilar approvals increased in 2022. Aduhelm is both costly and commonly produces side effects. months longer on the treatment.

pharmaphorum
JULY 6, 2022
— President Biden (@POTUS) July 7, 2022. Now, in addition to doctors and providers, the FDA announced pharmacists with your medical information can prescribe Paxlovid to patients.

PM360
AUGUST 9, 2022
The 2021 PURE report indicated that two out of three patients are concerned about potential side effects before taking their first dose, and again, this is more evident in women than men. Linda Morini & Emma Zheng, Adheris Health: “Medication Non-Adherence: It’s Not Just a New-to-Therapy Issue,” 2022, pg. May 23, 2018.

Pharmaceutical Technology
MAY 5, 2023
The FDA also warned against side effects with apetamin use similar to what is seen with antihistamine overdose, such as hallucinations, convulsions, coma and even death. Apetamin, in the form of tablets and syrup, is still available on several online sites, including stores on the popular marketplace Etsy.

pharmaphorum
JANUARY 6, 2023
In the phase 3 trial, TAK-755 reduced the incidence of thrombocytopenia events by 60% compared to plasma-based treatment and was much better tolerated, with less than 9% of patients on Takeda’s drug reporting side effects compared to almost half (48%) of those on standard therapy.
European Pharmaceutical Review
JANUARY 3, 2023
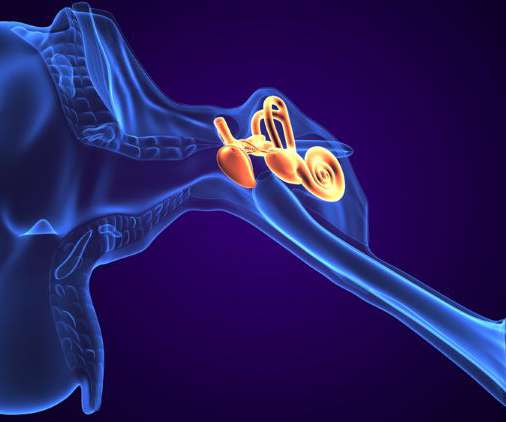
Sensorion, a biotechnology company has announced that the first patient has been enrolled in its NOTOXIS Phase IIa proof of concept clinical trial of SENS-401 (Arazasetron) in cancer patients experiencing cisplatin-induced ototoxicity (permanent hearing loss), a serious side effect of key chemotherapeutic agent cisplatin.

pharmaphorum
JANUARY 6, 2023
On 14th November 2022, Eli Lilly apparently wrote on its Twitter account, “We are excited to announce insulin is free now.” Add in more than 10,000 layoffs in healthtech since the start of 2022, and the pace of the industry has slowed considerably since January last year. Industry frustrations. New drug launches were delayed.
European Pharmaceutical Review
OCTOBER 20, 2022
These are all diseases that are highly prevalent in the population and are generally treated with drugs that are broadly immunosuppressive; while some of these drugs are beneficial and do ameliorate some symptoms, these patients experience side effects including increased susceptibility to infections. 5 Aug 2022. Gene Therapy.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content