Orchard’s MLD gene therapy becomes costliest US medicine
pharmaphorum
MARCH 20, 2024
Orchard Therapeutics has revealed the US price of Lenmeldy, its gene therapy for rare disease MLD, placing a $4.25m price tag on the one-shot treatment

 tag rare-diseases
tag rare-diseases 
pharmaphorum
MARCH 20, 2024
Orchard Therapeutics has revealed the US price of Lenmeldy, its gene therapy for rare disease MLD, placing a $4.25m price tag on the one-shot treatment

Pharmaceutical Technology
OCTOBER 17, 2023
The Netherlands-based company’s treatment has been awarded the designation by the US FDA following a successful Phase I trial.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Pharmaceutical Commerce
MARCH 6, 2024
Navigating the complex landscape of healthcare coverage can be an intimidating task, especially for patients with rare diseases for which treatments often come with a high price tag.

MedCity News
JULY 1, 2022
The $100 million price tag is in the neighborhood of the going rate for these vouchers, which grant a company a shorter regulatory review timeline for a drug that addresses a rare or neglected disease. Novartis is acquiring an FDA priority review voucher from Mallinckrodt Pharmaceuticals.

Pharmaceutical Technology
SEPTEMBER 30, 2022
Skysona is indicated as a one-time gene therapy to slow the progression of cerebral adrenoleukodystrophy (CALD), a rare paediatric neurodegenerative disease in boys aged 4–17 years diagnosed with early-stage CALD. Prior to bluebird's approvals, there were only two FDA-approved gene therapies for inherited conditions on the market.
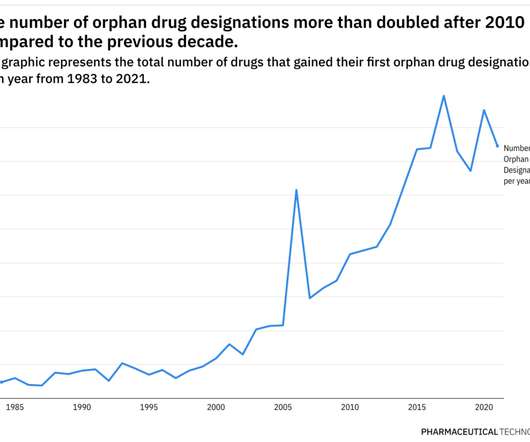
Pharmaceutical Technology
JUNE 29, 2022
This year has already been eventful when it comes to the development of therapies for rare diseases. Additionally, pricing and access for rare disease therapies continue to be scrutinized closely. Prior to the program, only 10 drugs were approved for a rare disease.

European Pharmaceutical Review
MAY 22, 2023
The review stated that therapeutic innovation has resulted in the production of expensive drugs to treat rare diseases, which need to be available on time. Smart contracts, which are essential for easing international trade and logistics operations, can enable the user to track fake returns to the producer and supplier using RFID tags.
Let's personalize your content