Potential blockbuster drugs to watch in 2023
European Pharmaceutical Review
JANUARY 10, 2023
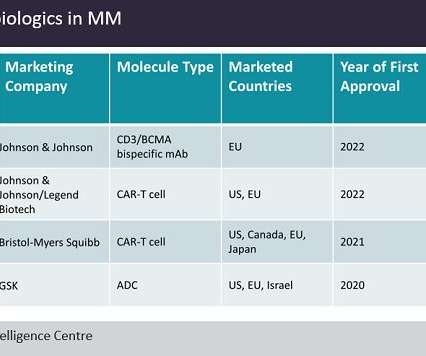
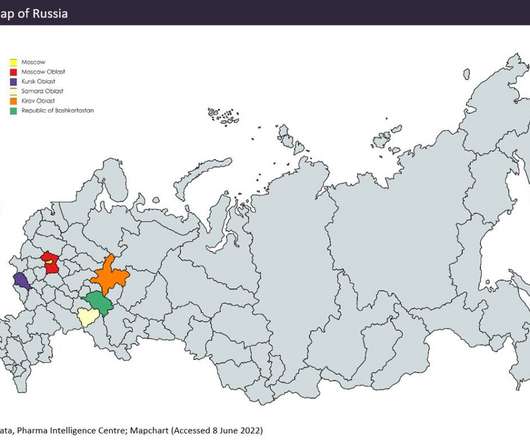
Clarivate Plc has released its Drugs to Watch 2023 report — among 70 of the drugs highlighted, including potential blockbuster drugs, the majority were revealed to be personalised medicines. The report offers predictive analysis of drugs entering the market or launching key indications in 2023.

































Let's personalize your content