Transforming pharmaceutical manufacturing: The AI revolution
European Pharmaceutical Review
JANUARY 9, 2024
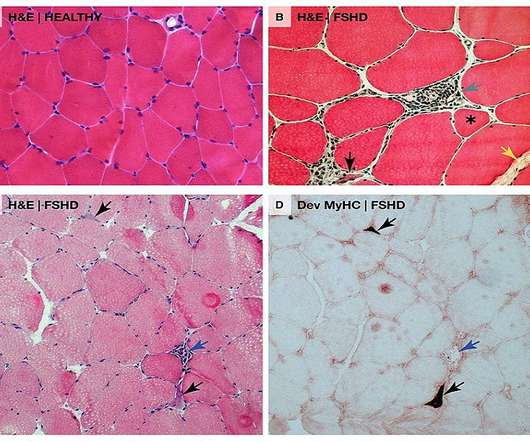
Revolutionising quality control In the backdrop of stringent quality standards and regulatory demands inherent to pharmaceutical manufacturing, the addition of AI technologies introduce a paradigm shift. AI and future perspectives: a glimpse into tomorrow Figure 2: adapted from Saha, G.

































Let's personalize your content