Metformin helps manage weight gain side effect of SGAs
European Pharmaceutical Review
OCTOBER 30, 2023
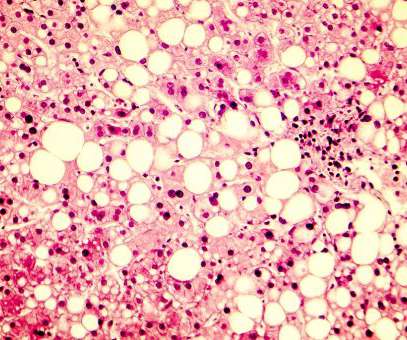
SGAs used to treat bipolar disorder are often effective at helping young patients’ mental health improve but can have significant side effects including elevated blood pressure and glucose, increased appetite, as well as weight gain.




































Let's personalize your content