FDA Commissioner: Insurers Need To Support Studies In Accelerated Drug Approval Pathway
MedCity News
MARCH 16, 2023
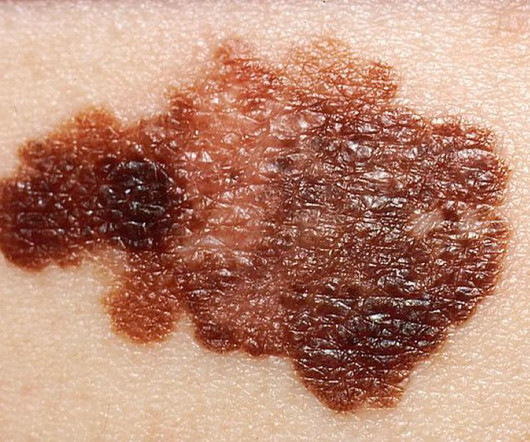
Physicians face a lot of barriers from insurers in completing studies on drugs in the accelerated approval pathway, declared Dr. Robert Califf, commissioner of food and drugs at the Food and Drug Administration. Insurers need to help, he said during the AHIP Medicare, Medicaid, Duals and Commercial Markets Forum.
































Let's personalize your content