Revolutionizing Pharmacovigilance with Proactive Signal Detection
PM360
NOVEMBER 27, 2023
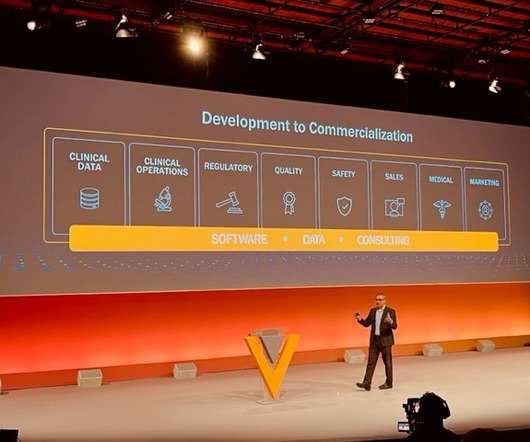
Up to now, drug safety teams have relied primarily on reactive reporting systems for pharmacovigilance (PV) or real-world drug safety monitoring. Most traditional signal detection methods focus on disproportionality, meanwhile calling attention to higher-than-expected correlations between the suspect drug and an adverse event (AE).































Let's personalize your content