J&J opens its wallet with whopping $8.9B talc settlement offer
Fierce Pharma
APRIL 4, 2023
J&J opens its wallet with whopping $8.

Fierce Pharma
APRIL 4, 2023
J&J opens its wallet with whopping $8.

Fierce Pharma
APRIL 6, 2023
Pfizer escapes proposed class action lawsuit over patient copay assistance esagonowsky Thu, 04/06/2023 - 11:03
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
eMediWrite
APRIL 6, 2023
Different types of video media are used in video marketing to advertise and market the goods and services offered by healthcare companies. An astounding 86% of healthcare companies use video marketing as a marketing strategy. The effectiveness of video as a marketing tool is also attributed to its extremely shareable nature. It tries to draw in and educate audiences, hold their attention, and turn them into customers.

MedCity News
APRIL 3, 2023
Hospitals are being more careful than ever when scrutinizing ROI for new technology. Ashis Barad — Allegheny Health Network’s chief information and digital officer — gave advice for health systems follow during the adoption process for new technology, such as ensuring clinicians are involved early on and viewing Big Tech as partners instead of threats.

PharmExec
APRIL 7, 2023
Eli Lilly debuts its first Mounjaro TV commercial amid high demand for the diabetes injection, which also shows promise in weight loss applications and outperformed rivals Ozempic and Wegovy in clinical trials.

Fierce Pharma
APRIL 3, 2023
After Johnson & Johnson loses again in bankruptcy case, it's game on for talc lawsuits kdunleavy Mon, 04/03/2023 - 06:44
Pharma Rep Focus brings together the best content for pharma rep professionals from the widest variety of industry thought leaders.

MedCity News
APRIL 2, 2023
Inato recently raised $20 million in Series A2 funding for its tech platform, which enables access to inclusive clinical trials. The two-way platform brings exposure to community-based providers who often are overlooked by Big Pharma for clinical trial sites by allowing providers to apply for clinical trials in which they’re interested.

PM360
APRIL 7, 2023
The idea of what constitutes the “point of care” is evolving. At its heart, the point of care has always revolved around the when and where patients are making decisions about their health. Often, these moments are happening in the presence of their healthcare provider (HCP), especially in-person at doctor’s offices. But now patients can also see an HCP via telehealth or chat with them through an online patient portal.

Fierce Pharma
APRIL 4, 2023
COVID-19 patent fights continue as Arbutus, Genevant come after Pfizer and BioNTech in new lawsuit zbecker Tue, 04/04/2023 - 11:01
Pharmaceutical Technology
APRIL 7, 2023
Enanta Pharmaceuticals has secured the US Food and Drug Administration’s (FDA) fast track designation for EDP-323 to treat respiratory syncytial virus (RSV). In vitro data of EDP-323 showed a significant reduction in RSV replication with picomolar potency in primary human bronchial epithelial cells against RSV A and B. Consistent potency was also observed across a range of RSV clinical isolates in several cell types.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

MedCity News
APRIL 2, 2023
Narcan, a nasal spray product that reverses the effects of opioid overdose, is now FDA approved for non-prescription use. Other recent FDA approvals include decisions for drugs from Pharming Group, Incyte, and Aurion Biotech.

InCrowd
APRIL 6, 2023
A well-known, established blockbuster brand at a top-10 pharma company needed a better solution for tracking its highly competitive market. Their traditional, data-heavy, time-consuming, and expensive ATU wasn’t meeting their needs. Learn how Essentials, InCrowd’s syndicated tracking solution, helped them collect competitive intelligence, save budgetary dollars, and eliminate their large ATU, allowing for more time and attention to the market events.

Fierce Pharma
APRIL 4, 2023
FDA authorizes InflaRx's anti-inflammation drug for most serious COVID despite phase 3 miss aliu Tue, 04/04/2023 - 16:48

Pharmaceutical Technology
APRIL 6, 2023
The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) has granted ADvantage Therapeutics’ immunotherapy AD04 an Innovation Passport for the treatment of Alzheimer’s disease. The designation, under the regulator’s Innovative Licensing and Access Pathway (ILAP), will fast-track a potential route to market for AD04 by providing collaborative opportunities with UK institutes like the National Institute for Health and Care Excellence (NICE).

Advertiser: ZoomInfo
Marketing technology is essential for B2B marketers to stay competitive in a rapidly changing digital landscape — and with 53% of marketers experiencing legacy technology issues and limitations, they’re researching innovations to expand and refine their technology stacks. To help practitioners keep up with the rapidly evolving martech landscape, this special report will discuss: How practitioners are integrating technologies and systems to encourage information-sharing between departments and pr

MedCity News
APRIL 4, 2023
Paris-based Sofinnova Partners announced last week that it has launched a new strategy aimed at investing in a new breed of startups that straddle the lines between data and biology.

Legacy MEDSearch
APRIL 6, 2023
Icentia Inc., announced that it has received FDA 510(k) clearance for CardioSTAT, an ambulatory, continuous ECG monitoring solution that relies on a wire free, single-use recorder. “This approval marks a key milestone for our company. The FDA clearance opens the door to the world’s largest medical device market. With the cost effectiveness and demonstrated ability of our cardiac monitoring solution to provide effective patient care and outcomes, we have no doubts that CardioSTAT will

Fierce Pharma
APRIL 6, 2023
Gilead caused injuries while waiting to develop safer HIV drugs, lawsuit claims zbecker Thu, 04/06/2023 - 11:40
Pharmaceutical Technology
APRIL 3, 2023
The European Commission (EC) has granted marketing authorisation for Sandoz’s biosimilar Hyrimoz (adalimumab) citrate-free high-concentration formulation (HCF). Hyrimoz has been approved for use in all the indications covered by the reference medicine Humira, including plaque psoriasis, rheumatic diseases, ulcerative colitis, Crohn’s disease, uveitis and hidradenitis suppurativa.

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.

MedCity News
APRIL 6, 2023
As a first step to move toward greater hospital price transparency and lower costs, CMS can immediately issue $2 million fines on the 30% of American hospitals it concludes are noncompliant. If CMS takes enforcement seriously, the hospital industry will respond by quickly coming into compliance.

Copyright Clearance Center
APRIL 4, 2023
The following is an excerpt from Accessing and Analyzing Relevant Content in Today’s Information Chaos. Semantic enrichment is the ingredient behind getting relevant search results even if they don’t use the same terminology as the query. For example, a query for “rare disease drug approval” would include results for the Orphan Drug Act from the FDA.

Fierce Pharma
APRIL 3, 2023
After rumored merger fell through, Merck and Seagen's Padcev-Keytruda combo wins bladder cancer nod fkansteiner Mon, 04/03/2023 - 18:10
Pharmaceutical Technology
APRIL 4, 2023
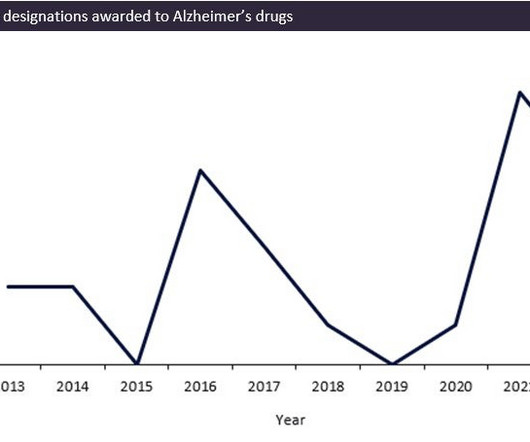
The FDA has seen a record surge in review designations being awarded over the last two years for Alzheimer’s indications, with 12 review designations being awarded to drugs between 2020 and 2022. This coincided with the much-anticipated wave of monoclonal antibody drugs for Alzheimer’s disease (AD) such as Eisai/Biogen’s Leqembi (lecanemab-irmb) and Eli Lilly’s donanemab, which are predicted to provide significant improvement on previous AD therapies.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

MedCity News
APRIL 5, 2023
Consumers are demanding the constant need for technology that is simple, connected and convenient, and medtech companies have had to accelerate to meet those needs. They are embracing these changing times and using it as an opportunity to innovate in three key areas.

PM360
APRIL 7, 2023
PM360 asked experts in developing market access strategies about how the Inflation Reduction Act will impact drug pricing negotiation and other access-related issues as well as what other big trends in the space will force companies to rethink their strategies. Specifically, we asked them: What impact should market access professionals expect from the Inflation Reduction Act (IRA)?

Fierce Pharma
APRIL 4, 2023
Pfizer moves on up to the West Side, establishing new nerve center at Hudson Yards' Spiral skyscraper fkansteiner Tue, 04/04/2023 - 11:42

Pharmaceutical Technology
APRIL 3, 2023
Sartorius , through its French listed sub-group Sartorius Stedim Biotech , has signed an agreement to acquire Polyplus for €2.4bn ($2.6bn). The deal will see Polyplus join the German life science group’s portfolio allowing the latter to leverage expertise in transfection reagents and plasmid DNA for gene therapy. Polyplus, based in Strasbourg, France, produces key components in the production of viral vectors used in cell and gene therapies.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.

MedCity News
APRIL 2, 2023
By supporting next-generation payment methods that boost security and convenience for consumers, providers can enhance patient satisfaction and loyalty.

PharmaVoice
APRIL 6, 2023
Pharming just landed an FDA approval for a treatment targeting an ultra-rare immunodeficiency disorder discovered only a decade ago — and it’s already in the hands of patients.

Fierce Pharma
APRIL 5, 2023
AstraZeneca says Lynparza, Imfinzi combo excels in first look at ovarian cancer trial kdunleavy Wed, 04/05/2023 - 10:46
Pharmaceutical Technology
APRIL 3, 2023
BioNTech has signed exclusive licence and collaboration agreements with Duality Biologics (DualityBio) for the development of two antibody-drug conjugate (ADC) assets for solid tumours. The agreements also include the manufacturing and commercialisation of the two assets, including DB-1303 and DB-1311, across the globe. As part of the new deals, BioNTech will have commercial rights to the ADCs worldwide, excluding mainland China, the Hong Kong special administrative region and the Macau special

Advertiser: ZoomInfo
In times of economic uncertainty, account-based strategies are essential. According to several business analysts and practitioners, ABM is a necessity for creating more predictable revenue. Research shows that nearly three-quarters of marketers (74%) already have the resources needed to build successful ABM programs.
Let's personalize your content