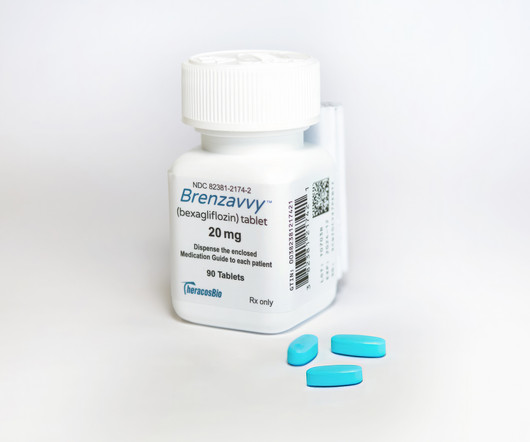
TheracosBio teams with Mark Cuban's Cost Plus to launch Brenzavvy at bargain price
Fierce Pharma
JULY 12, 2023
As another SGLT2 diabetes drug hits the market, the obvious question is: How will it find a way to compete against formidable blockbusters Farxiga and Jardiance? | TheracosBio and Mark Cuban's online distributor Cost Plus Drug Company have partnered to provide newly approved SGLT2 inhibitor Brenzavvy at a major discount to competitors in the class, including AstraZeneca's Farxiga and Eli Lilly and Boehringer Ingelheim's Jardiance.






































Let's personalize your content