BMS settles lawsuit with two fired employees who refused COVID vaccines
Fierce Pharma
JANUARY 26, 2023
BMS settles lawsuit with two fired employees who refused COVID vaccines kdunleavy Thu, 01/26/2023 - 14:30

Fierce Pharma
JANUARY 26, 2023
BMS settles lawsuit with two fired employees who refused COVID vaccines kdunleavy Thu, 01/26/2023 - 14:30

MedCity News
JANUARY 26, 2023
A survey from The Leapfrog Group asked employers to give their health plans an “A” through “F” letter grade. From those letter grades, Leapfrog calculated the health plans’ average Grade Point Average (GPA) on a 4.0 scale. Health plans received a 2.29 GPA in 2022, a slight decrease from Leapfrog’s prior survey in 2020, which had a 2.57 GPA.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Fierce Pharma
JANUARY 26, 2023
Fierce Pharma Asia—Takeda's cancer deal; BeiGene's Brukinsa nod; Astellas' gene therapy reprieve aliu Thu, 01/26/2023 - 14:58

MedCity News
JANUARY 26, 2023
Tia — a startup providing hybrid primary care, OB/GYN services and mental health care to women — is now partnered with Cedars-Sinai in addition to UCSF Health and CommonSpirit Health. Under Tia’s partnership model, the company combines its primary care with health systems’ specialty care to reduce fragmentation and advance preventive health.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Fierce Pharma
JANUARY 26, 2023
Abortion pill maker GenBioPro sues West Virginia over abortion ban zbecker Thu, 01/26/2023 - 14:38

MedCity News
JANUARY 26, 2023
Pearl Health — a technology company that helps independent physician practices participate in value-based care models — just raised $75 million in Series B financing. Pearl’s platform leverages data science to help primary care providers focus on patients who are driving expenses and need care the most.
Pharma Rep Focus brings together the best content for pharma rep professionals from the widest variety of industry thought leaders.

MedCity News
JANUARY 26, 2023
The $58 million funding round was led by Portage and included participation from PruVen Capital, Wing Venture Capital and others. With the funding, Angle Health plans to expand into additional markets and grow its membership.

Fierce Pharma
JANUARY 26, 2023
Understanding the Need for Rigorous Independent Adjudication mteefey Thu, 01/26/2023 - 15:58

MedCity News
JANUARY 26, 2023
It’s time to stop thinking about prior authorizations as a transaction. Rather, PA is the start of a care episode that should be a smooth, well-coordinated journey for patients, their providers and their health plan.

European Pharmaceutical Review
JANUARY 26, 2023
Yescarta ® ▼(axicabtagene ciloleucel; axi-cel) is now the first chimeric antigen receptor (CAR) T-cell therapy and first personalised immunotherapy to be recommended for routine use on the NHS in England. This is based on final draft guidance from the National Institute for Health and Care Excellence (NICE). The therapy by Gilead Sciences and Kite is indicated for eligible adults with diffuse large B-cell lymphoma (DLBCL) and primary mediastinal large B-cell lymphoma (PMBCL) who have already bee

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
MedCity News
JANUARY 26, 2023
Miach Orthopaedics — a medical device company focused on restoring torn ACLs instead of reconstructing them — recently received $40 million in funding. The capital will be used to expand the U.S. commercial rollout of the company’s implantable device, which received FDA de novo clearance in 2020.

pharmaphorum
JANUARY 26, 2023
2022 was a banner year for genomics. In March, the collaborative T2T consortium published the first complete telomere-to-telomere sequence of the human genome, filling in the last 8% of the 3 billion base pairs that make up our DNA. And in the UK specifically, genomics remained high on the national agenda, with several significant government programmes and investments announced – including the Newborn Genomes Programme in healthcare and the Precision Breeding Bill in the agricultural sector.

Fierce Pharma
JANUARY 26, 2023
Bristol Myers makes case for Breyanzi in chronic lymphocytic leukemia. Will it make a difference?

Pharmaceutical Technology
JANUARY 26, 2023
Health Canada has accepted for review Valeo Pharma partner Veru’s new drug submission (NDS-CV) for Covid-19 therapy sabizabulin. The new dual antiviral and anti-inflammatory agent Sabizabulin is being developed to treat hospitalised adult patients with moderate to severe Covid-19 who are at high acute respiratory distress syndrome (ARDS) and mortality risk.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

European Pharmaceutical Review
JANUARY 26, 2023
Typical extractables and leachables (E/L) studies aim to identify, quantify and ultimately minimise any impurities that can migrate from packaging into a final product or drug. Gas or liquid chromatography-mass spectrometry (LC/MS) or (GC/MS) have been widely used in extractables studies however, there are several challenges associated with E&L analyses including: Detection and quantification of unknowns Sensitivity and detection at trace levels Reducing uncertainty factors Increasing s

pharmaphorum
JANUARY 26, 2023
Guidance on the way pharma companies communicate about prescription medicines has been updated in the UK to cover use of social media channels for the first time. The document from the Prescription Medicines Code of Practice Authority (PMCPA) arrives in the wake of several cases where drugmakers made promotional statements on social media platforms – including LinkedIn and Instagram – that were deemed to have breached the code of practice laid down by the Association of British Pharmaceutical In

European Pharmaceutical Review
JANUARY 26, 2023
The European Medicines Agency (EMA) has published an updated Q&A document regarding ICH M10 ‘Bioanalytical Method Validation and Study Sample Analysis’. The ICH M10 guideline provides recommendations on the validation of bioanalytical assays for chemical and biological drugs and their metabolites in biological matrices. These concentration measurements are used as part of regulatory decisions regarding the safety and efficacy of medicinal products.

Pharma Pathway
JANUARY 26, 2023
Rallis India Limited-Walk-In Interview foe Production/ QA/ QC On 29th Jan’ 2023 Job Description Walk-In Interview foe Production/ QA/ QC On 29th Jan’ 2023 @ Rallis India Limited Department: Production Designation: Associate Qualification: Diploma Chemical, M.Sc (Chemistry), B.Sc (Chemistry) Experience: 03 to 10 years Must have experience of agro Chemical plant.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.
European Pharmaceutical Review
JANUARY 26, 2023
Enhertu (trastuzumab deruxtecan) has been approved in the European Union (EU) for adults with unresectable or metastatic HER2-low (IHC 1+ or IHC 2+/ISH-) breast cancer. The approval is indicated for those who have received prior chemotherapy in the metastatic setting or developed disease recurrence during or within six months of completing adjuvant chemotherapy.

Pharma Pathway
JANUARY 26, 2023
Strides Pharma -Walk-In Drive for Quality Control/ Production / Packing /Maintenance Engineer On 29th Jan’ 2023 Job Description Walk-In Drive for Quality Control/ Production / Packing /Maintenance Engineer On 29th Jan’ 2023 @ Strides Pharma Department: Quality Control (Formulations) Qualification: M.Sc/ B.Pharm/ M.Pharm Experience: 02-05 years Work Location: Bangalore/ Pondicherry Department: Production / Packing (Tablet & Capsules) Qualification: B.Pharm/ M.Pharm Experience:

pharmaphorum
JANUARY 26, 2023
Roche and Boehringer Ingelheim have both formed partnerships with tech companies this week to develop and deploy wearable biosensor technology for use in clinical trials and patient monitoring. Roche’s alliance with France’s Sysnav Healthcare is in the area of movement disorders, and will build on the tech company’s wearable magneto-inertial sensor device for 3D movement tracking, which has already been accepted as a digital endpoint for use in Duchenne muscular dystrophy (DMD)
Pharma Pathway
JANUARY 26, 2023
Hetero Labs Limited-Walk-In Interviews for Freshers in QC/ QA/ R&D/ Production/ HR/ Maintenance On 29th Jan’ 2023 Job Description Hetero Labs Limited is one of India’s generic pharmaceutical companies and the “world’s largest producer of anti- retroviral drugs”. Hetero’s business includes APIs, generic, biosimilars, custom pharmaceutical services, and branded generics.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.

PharmaTimes
JANUARY 26, 2023
Trial will evaluate efficacy of anti-netrin-1 antibody candidate NP137 in combination with Atezo-bev
Pharma Pathway
JANUARY 26, 2023
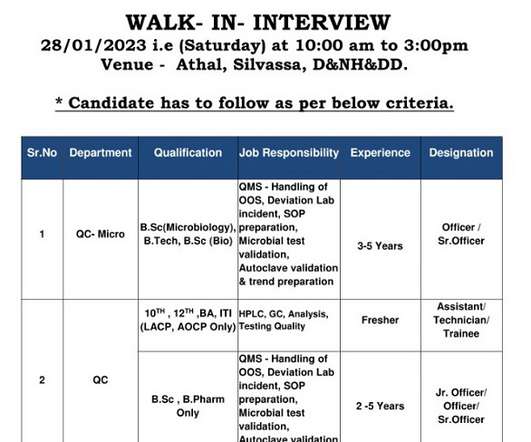
Ipca Laboratories Ltd- Walk-In Interview for Freshers & Experienced in QC/ QA/ QC-Micro On 28th Jan’ 2023 Job Description Walk-In Interview for Freshers & Experienced in Quality Control/ Quality Assurance/ QC-Micro @ Ipca Laboratories Ltd Department: Quality Control/ Quality Assurance/ QC-Micro Posts: Trainee/ Officer/ Sr. Officer/ Assistant/ Technician Qualification: B.Sc (Microbiology), B.Tech, B.Sc (Bio), 10th, 12th, BA, ITI (LACP, AOCP Only), B.Pharm Experience: 02-05 years/
pharmaphorum
JANUARY 26, 2023
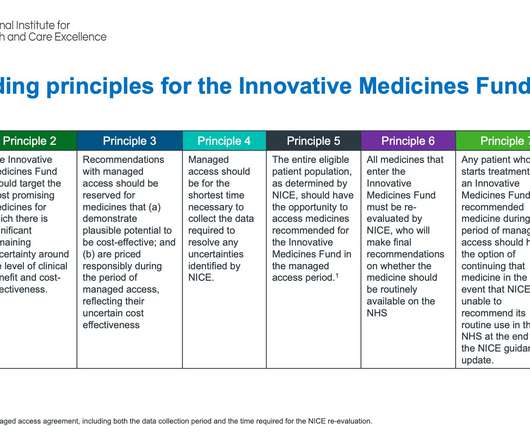
In June 2022, NHS England (NHSE) and the National Institute for Health and Care Excellence (NICE) launched the Innovative Medicines Fund (IMF). The £340 million fund will provide non-cancer patients access to cutting-edge treatments that still have uncertainties at the time of launch and promotes the UK as an attractive launch destination. The IMF was launched one month before the NHS shifted to a whole system approach, through the formal implementation of Integrated Care Systems (ICSs).

Pharma Pathway
JANUARY 26, 2023
Rallis India Limited-Walk-In Interview for Production/ QA/ QC On 29th Jan’ 2023 Job Description Walk-In Interview for Production/ QA/ QC On 29th Jan’ 2023 @ Rallis India Limited Department: Production Designation: Associate Qualification: Diploma Chemical, M.Sc (Chemistry), B.Sc (Chemistry) Experience: 03 to 10 years Must have experience of agro Chemical plant.

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic
pharmaphorum
JANUARY 26, 2023
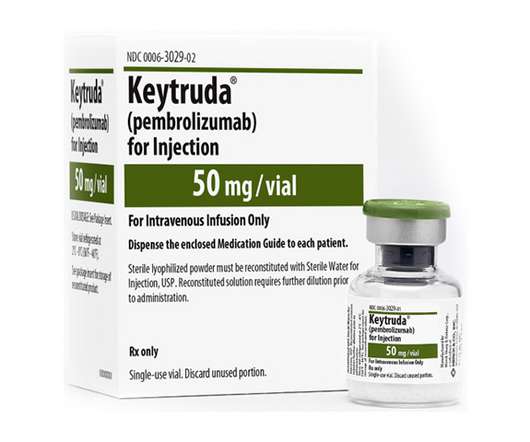
Prostate cancer has proved an elusive target for cancer immunotherapies, and Merck & Co’s checkpoint inhibitor Keytruda is the latest to fall short in the disease in a phase 3 trial. Merck has said it will abandon the KEYNOTE-991 study of PD-1 inhibitor Keytruda (pembrolizumab) in patients with metastatic hormone-sensitive prostate cancer (mHSPC) after it showed no benefit on either overall survival (OS) or radiographic progression-free survival (rPFS) when the data was analysed midway

Pharma Pathway
JANUARY 26, 2023
Sun Pharmaceutical Industries Ltd- Virtual Walk-In Drive for R&D Biotechnology On 29th Jan’ 2023 Job Description Company: Sun Pharmaceutical Industries Ltd, Baroda Openings for R&D Biotechnology Department: R&D Biotechnology-In process Analysis Education: M.Sc / M.Tech/ MS. Pharm in Biotechnology/ Biochemistry Experience: 02 to 05 years in R&D Brief Job Description: The Candidates is expected to develop and qualify the HPLC based methods for protein and peptide products.

PharmaTimes
JANUARY 26, 2023
Treatment involves adult patients with relapsed or refractory diffuse large B-cell lymphoma

Pharma Pathway
JANUARY 26, 2023
Annora Pharma -Walk-In Interview for Freshers & Experienced in Production-Mfg/ Packing On 27th Jan’ 2023 Job Description Walk-In Interview for Freshers & Experienced in Production-Mfg/ Packing On 27th Jan’ 2023 @ Annora Pharma Departments: Production-Mfg/ Packing Positions: Officer/ Operator/ Executive Qualification: B.Sc / M.Sc/ B.Pharm/ B.Tech/ Diploma/ ITI Experience: 0 to 04 years Location: Hyderabad Date : 27th Jan’ 2023 Time: 09:00 AM to 02:00 PM Venue: Annora

Advertisement
The global landscape of clinical trials is rapidly changing as studies become more complex. An increasing number of sponsors are seeking enhanced flexibility in their supply chains to address a variety of clinical supply challenges, including patient demand and reducing delays. Demand-led supply and direct-to-patient distribution are next-generation solutions that are helping to meet these growing needs, allowing for more streamlined processes and patient-centric studies.
Let's personalize your content