After FDA snub, Daiichi's Vanflyta gains US approval and tees up AML clash with Novartis, Astellas
Fierce Pharma
JULY 21, 2023
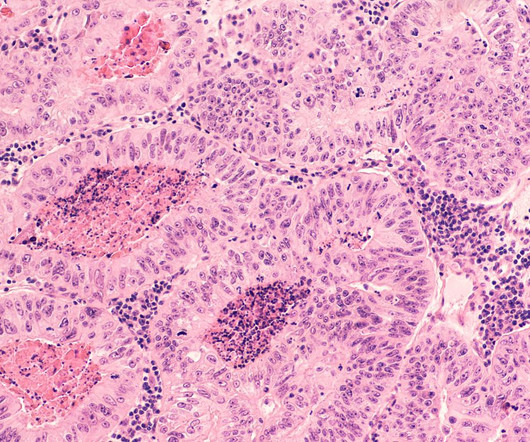
If at first you don’t succeed, try, try again. That’s the lesson Daiichi Sankyo likely took home Friday after scoring a long-awaited U.S. | Some four years after Daiichi Sankyo's quizartinib scored its initial green light in Japan—and suffered a slap-down from drug regulators stateside—the acute myeloid leukemia medicine has won the FDA’s blessing.






































Let's personalize your content