AstraZeneca says Lynparza, Imfinzi combo excels in first look at ovarian cancer trial
Fierce Pharma
APRIL 5, 2023
AstraZeneca says Lynparza, Imfinzi combo excels in first look at ovarian cancer trial kdunleavy Wed, 04/05/2023 - 10:46

Fierce Pharma
APRIL 5, 2023
AstraZeneca says Lynparza, Imfinzi combo excels in first look at ovarian cancer trial kdunleavy Wed, 04/05/2023 - 10:46

MedCity News
APRIL 5, 2023
Consumers are demanding the constant need for technology that is simple, connected and convenient, and medtech companies have had to accelerate to meet those needs. They are embracing these changing times and using it as an opportunity to innovate in three key areas.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Fierce Pharma
APRIL 5, 2023
GSK will have to pay additional royalties to AstraZeneca on cancer drug Zejula kdunleavy Wed, 04/05/2023 - 17:03

MedCity News
APRIL 5, 2023
Avia CEO Linda Finkel has had many conversations with health systems executives about why their technology initiatives haven’t gone as planned in the past. Based on these experiences, she has noticed four main reasons hospitals don’t see the results for which they were hoping — including not exercising enough scrutiny during the vendor selection process and failure to think about capability at scale.

Fierce Pharma
APRIL 5, 2023
Bayer's incoming CEO Anderson won't rule out consumer, agriculture spinoffs: report fkansteiner Wed, 04/05/2023 - 10:35
MedCity News
APRIL 5, 2023
The FDA granted emergency use authorization to an InflaRx antibody drug that treats hospitalized Covid-19 patients. But Pardes Bioscience’s coronavirus journey is ending following the Phase 2 failure of an antiviral that was expected to compete with Pfizer’s Paxlovid.
Pharma Rep Focus brings together the best content for pharma rep professionals from the widest variety of industry thought leaders.

Pharmaceutical Technology
APRIL 5, 2023
Austrian molecular glue degraders discovery and development company Proxygen and Merck , known as MSD outside the US and Canada, have entered into a multi-year research collaboration and licence deal. Through the collaboration, the companies will work together to identify and develop molecular glue degraders against multiple therapeutic targets. MSD will make an upfront payment to Proxygen, which will also be eligible for up to $2.55bn in future payments, subject to the achievement of agreed res

MedCity News
APRIL 5, 2023
To address the burden faced by family caregivers across the country, Tomorrow Health CEO Vijay Kedar argues that the U.S. healthcare system should expand its reimbursement for the care they provide. Not only is caregiving physically and emotionally taxing, but it can also cause severe financial strain —many caregivers take time off work and spend their own money to support their loved ones’ care, he explained.

Pharmaceutical Technology
APRIL 5, 2023
The US Food and Drug Administration (FDA) has granted emergency use authorisation (EUA) to InflaRx’s Gohibic (vilobelimab) to treat critically ill hospitalised Covid-19 adult patients. Gohibic is indicated for use in these patients within 48 hours of receiving invasive mechanical ventilation (IMV) or extracorporeal membrane oxygenation (ECMO). It is a first-in-class monoclonal anti-human complement factor C5a antibody.

MedCity News
APRIL 5, 2023
Preventing drug diversion starts with understanding the signs of a substance use disorder (SUD) in hospital and health system staff workers, those closest to the problem and one of the most affected parties.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Copyright Clearance Center
APRIL 5, 2023
The post CCC Named to KMWorld 100 Companies That Matter in Knowledge Management for 2023 appeared first on Copyright Clearance Center.

MedCity News
APRIL 5, 2023
With the growing complexity of identity management and the challenges posed by market consolidation and digital front door initiatives, it is essential to implement a solution that will effectively manage patient information and prevent duplicates and identity issues.

European Pharmaceutical Review
APRIL 5, 2023
According to a market report , the global cell and gene therapy (CGT) supply chain/ logistics market is expected to reach $3.12 billion in 2031. This translates into a promising compound annual growth rate (CAGR) of 11.2 percent during the forecast period of 2023-2031. What is driving the cell and gene therapy supply chain/logistics market? The growing need for cell and gene treatments, growing medical trials and improvements in real-time supply chain operations are predicted to enhance the qual

PharmaTimes
APRIL 5, 2023
Individual receives AVA6000 treatment as part of additional dose escalating study

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
Pharmaceutical Technology
APRIL 5, 2023
More than a decade after the first patient was treated with a CAR-T therapy, six therapies relying on the same principles have been approved by the US Food and Drug Administration (FDA) and marketed to thousands of patients. But access to these treatments continues to remain limited due to high price tags and variable availability across regions. Currently, all approved CAR-T therapies are autologous, where T cells taken from patients are modified and then re-infused.

PharmaTimes
APRIL 5, 2023
Company disappointed by institute’s failure to recommend thyroid cancer therapy for specific patient group
Pharmaceutical Technology
APRIL 5, 2023
Caribou Biosciences has secured fast track designation from the US Food and Drug Administration (FDA) for its CAR-T cell therapy, CB-011. The therapy is currently under development for the treatment of patients with relapsed or refractory multiple myeloma (r/r MM). Caribou is presently conducting its Phase l CaMMouflage trial to assess CB-011 in r/r MM patients.

Spotio
APRIL 5, 2023
Listen to the episode on Spotify. Chris Pierce is a mental toughness expert who trains sales professionals to double and triple their results. He’s here to help you learn how to do the same. _ Guest At A Glance Name : Chris Pierce What He Does: As a mental toughness expert, Chris has a proven track record of empowering sales professionals to double and even triple their productivity.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.

Clarify Health
APRIL 5, 2023
The cost and quality of providers vary widely, even within a single specialty in a single market. Where patients get their medical care directly impacts the cost of care and clinical outcomes. Physicians are often in the driver’s seat when it comes to site of service decisions—they’re making referrals for patients who need to see a specialist or go to a facility.

PM360
APRIL 5, 2023
The Point of Care Marketing Association (POCMA), a non-profit organization that aims to support the continued growth of the Point of Care (POC) media channel, hosted 270 healthcare marketers at its annual POC NOW Industry Summit at City Winery in New York City , March 22 nd. For the event’s inaugural POC Excellence Awards, POCMA gathered a panel of independent industry experts to review entries across four categories.

Quantified
APRIL 5, 2023
In today’s tumultuous job market, finding and retaining top talent is an ongoing priority for most companies. That’s especially true in sales: average turnover among sales reps is almost triple that of other occupations. Tragically, the seeds of disaffection are often planted at the very beginning of a rep’s tenure in the organization.

Pharma Marketing Network
APRIL 5, 2023
Attending the Reuters Pharma Marketing 2023 event on March 28-29 was a fascinating experience as the pharma marketing industry continues to return to in-person events. The event included a focus on Medical Affairs, as a reflection of industry moving away from a ‘church and state’ approach to medical and commercial. We also saw the addition of a track focused on Cell and Gene Therapy, as Pharma ventures into the commercialization of therapeutics.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

PharmaVoice
APRIL 5, 2023
Welcome to the Woman of the Week podcast, a weekly discussion that illuminates the unique stories of women leaders who are catalyzing change throughout the life sciences industry. You can check out all our podcast episodes here. Dr. Theresa Heah has spent her entire career moving ophthalm.

Prognos Health
APRIL 5, 2023
Todd Somsel , Commercial Lead, RWD Marketplace As I reflect on the 2023 edition of the Reuters Pharma conference in Philadelphia last week, I continue to be motivated by the excitement behind the many uses of real-world data (RWD) and the large number of data and service providers growing their offerings in this space. At the same time, the RWD landscape remains tremendously challenging, which threatens to limit the true potential of this rich data source.
Pharmaceutical Technology
APRIL 5, 2023
AnaBios has strengthened its human tissue and cells portfolio with the purchase of Cell Systems for an undisclosed sum. Based in Kirkland, Washington, US, Cell Systems is a human primary cell and cell culture media company. The company provides several human primary cells, including endothelial cells from the brain, liver, kidney, eye and lung which facilitate the ongoing evaluation of Covid-19.

Pharma Leaders
APRIL 5, 2023
UK-based oncology therapeutics company Mosaic Therapeutics has raised $28m from series A funding round. It also announced the appointment of former Novartis Oncology SVP Brian Gladsden as CEO. The firm raised the amount from Syncona Investment Management, and Cambridge Innovation Capital. Proceeds from the Series A funding round will be used to advance Mosaic’s pipeline of targeted oncology therapies for biomarker-stratified populations, advancing its lead programmes via preclinical development

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic

Pharmaceutical Technology
APRIL 5, 2023
Luye Pharma Group has signed a marketing and distribution agreement with Duopharma Biotech’s wholly-owned subsidiary, Duopharma Marketing, for a cholesterol management product in Malaysia. Duopharma Marketing will have exclusive rights to market and distribute a traditional Chinese medicine, developed by Luye Pharma for cholesterol management. Duopharma Biotech Group managing director Leonard Ariff Abdul Shatar said: “This partnership and collaboration with Luye Pharma enables all patients in Ma
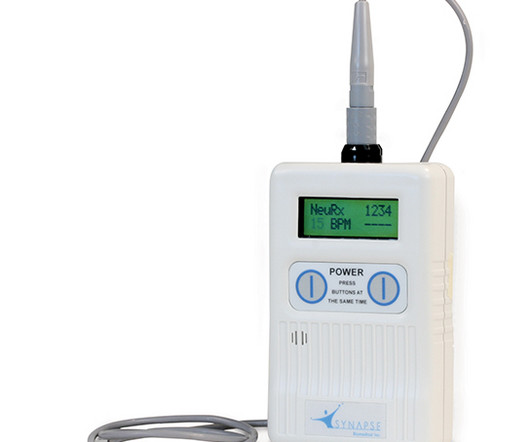
Legacy MEDSearch
APRIL 5, 2023
Synapse Biomedical, Inc. announced today that the FDA has granted premarket approval (PMA) of the NeuRx ® Diaphragm Pacing System (NeuRx DPS ® ) for use in patients with spinal cord injuries who rely on mechanical ventilation. PMA is the most stringent type of device marketing application required by FDA. More hospitals are expected to begin implementing the NeuRx DPS ® now that they no longer have to undergo the lengthy internal review and approval process required under the previous humanitari
Pharmaceutical Technology
APRIL 5, 2023
The European Medicines Agency (EMA) has announced plans to expedite scientific advice and support the preparation of regulatory packages under its PRIority Medicines (PRIME) scheme. PRIME is an EMA scheme to support the development of medicines for rare diseases and conditions with high unmet need. Last year, the EMA approved 49 new medicines , of which eight were recommended for approval through the PRIME scheme.

PharmExec
APRIL 5, 2023
Kevin Hagan, president and CEO of the PAN Foundation, spoke to PharmExec about the recent IRA changes to Medicare and how it positively effects patients.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content