MHRA consults public on reclassification of codeine linctus
Pharmaceutical Technology
JULY 18, 2023
MHRA is consulting health professionals and the public on the potential reclassification of codeine linctus to a prescription-only medicine.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

Pharmaceutical Technology
JULY 18, 2023
MHRA is consulting health professionals and the public on the potential reclassification of codeine linctus to a prescription-only medicine.

Pharmaceutical Technology
MARCH 19, 2024
Eli Lilly's LillyDirect allows for online consultations and prescriptions, and Amazon delivers medicines to the patient’s home.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
European Pharmaceutical Review
APRIL 30, 2024
The Committee for Human Medicinal Products (CHMP) has recommended the approval of new eight medicines. The committee also recommended the biosimilar medicine Wezenla (ustekinumab) to treat of plaque psoriasis. Last week, the US FDA approved a new gene therapy for eligible adults with haemophilia B.
Contrarian Sales Techniques
FEBRUARY 24, 2023
Do your own research and consult with experts when making decisions about your healthcare. When buying medicines, it is important to be cautious of counterfeit products and to do your research before purchasing to ensure that you are getting quality products.

European Pharmaceutical Review
JANUARY 27, 2023
The UK will be the first country to introduce a tailored framework for the regulation of innovative medicines manufactured at the point where a patient receives care, the Medicines and Healthcare products Regulatory Agency (MHRA) has announced.

European Pharmaceutical Review
DECEMBER 19, 2023
The European Medicines Agency (EMA) and the Heads of Medicines Agencies (HMAs) have published a plan, which sets out a “collaborative and coordinated strategy” to maximise the benefits of artificial intelligence in regulation. The plan was prepared by the Big Data Steering Group (BDSG), a joint initiative between HMA and EMA.

European Pharmaceutical Review
NOVEMBER 16, 2023
However, hundreds of thousands of patients each year are missing out on medicines with a proven track record of being effective and safe,” Zorana Maravic, CEO of Digestive Cancers Europe and Act4Biosimilars Steering Committee member commented. Act4Biosimilars is supported by its founding sponsor, Sandoz.

European Pharmaceutical Review
FEBRUARY 8, 2024
Vertex scores European cystic fibrosis medicine approval Efficacy of the vanzacaftor/tezacaftor/deutivacaftor (vanza triple) Vertex also shared that in the SKYLINE 102 and SKYLINE 103 , head-to-head against TRIKAFTA, for first key secondary endpoint, the vanza triple was found to be superior in reducing sweat chloride (SwCl) levels.

European Pharmaceutical Review
SEPTEMBER 28, 2023
According to the European Federation of Pharmaceutical Industries and Associations (EFPIA), more than 600 essential medicines are at risk if a proposed restriction on the use of fluorinated substances, per- and polyfluoroalkyl substances (PFAS) across pharmaceutical manufacturing in the EEA, is implemented.

Medico Reach
DECEMBER 2, 2022
To bring effective medicines and Innovations in healthcare industry , a Clinical Research Organization or Contract Research Organization (CRO) are a must for the Biotech, Medtech, and pharma industries. Moreover, it works hard to speed up medicine development operations to introduce better and more effective medicine sooner in the market.

European Pharmaceutical Review
JULY 21, 2023
European Medicines Agency (EMA) has published a draft reflection paper on using artificial intelligence (AI) to support the safe and effective development, regulation and use of medicines. How can AI and ML support development and regulation of medicines?

European Pharmaceutical Review
DECEMBER 12, 2022
The Scottish Medicines Consortium (SMC) has accepted Utrogestan, the only adjunctive micronised progesterone available as hormone replacement therapy (HRT) for use alongside any oestrogen-only HRT for women with an intact uterus. Adjunctive micronised progesterone – a menopause milestone for Scottish women.

PM360
FEBRUARY 23, 2024
The campaign highlighted BeiGene’s commitment to high-quality, accessible medicines, as well as its robust clinical trial program, oncology products, and pipeline. An omnichannel mix of promotions enhanced BeiGene’s reputation and gave account directors effective communication tools.

pharmaphorum
NOVEMBER 23, 2022
Harking back to the Reuters Pharma 2022 event, held at the Nice Acropolis convention centre on France’s South Coast back in October, pharmaphorum had a chance to drop by the booth of EY and discuss not only its consulting operations, but the company’s Smart Reviewer, and their own assessment of the industry event itself.

European Pharmaceutical Review
DECEMBER 12, 2022
The Scottish Medicines Consortium (SMC) has approved Utrogestan, the only adjunctive micronised progesterone available as hormone replacement therapy (HRT) for use alongside any oestrogen-only HRT for women with an intact uterus. Adjunctive micronised progesterone – a menopause milestone for Scottish women.

European Pharmaceutical Review
MARCH 24, 2023
The European Medicines Agency (EMA) has published a report summarising the mid-point achievements of its Regulatory Science Strategy (RSS) to 2025. Regulatory science refers to the scientific disciplines that are applied to the quality, safety and efficacy assessment of medicinal products.
European Pharmaceutical Review
NOVEMBER 6, 2023
A new medicine to treat adults with non-small cell lung cancer (NSCLC) with a rare mutation called KRAS G12C has been authorised by the UK Medicines and Healthcare products Regulatory Agency (MHRA). According to the MHRA, Mirati Therapeutics B.V.’s

Pharmacy Times
NOVEMBER 14, 2022
When it comes to individualized treatment, “we can do better,” said John Edwards, vice president of Healthcare Solutions Consulting at SoftServe.

European Pharmaceutical Review
DECEMBER 13, 2022
David Elder (David P Elder Consultancy). Clinical innovation: using digital solutions to deliver the next wave of medicines. Included in this issue of European Pharmaceutical Review : . The lingering menace of diethylene glycol / ethylene glycol adulteration. SUSTAINABILITY. Stefan Koenig, Martin Olbrich and Jan Backmann, Roche.

Clarivate
FEBRUARY 5, 2024
Here are some key takeaways: AI and machine learning will have applications throughout every phase and aspect of drug development and will open up previously unreachable frontiers of medicine. “AI Payers, too, will be challenged by AI- and machine learning-enabled advances in medicine. s Artificial Intelligence Act and the U.S.
Pharmaceutical Technology
FEBRUARY 23, 2023
GenScript ProBio has announced a strategic collaboration with RVAC Medicines to manufacture GMP-grade plasmid DNA (pDNA) for the latter’s RVM-V001, an mRNA Covid-19 vaccine candidate. GenScript ProBio CEO Dr Brian Min said: “We are delighted to enter into this strategic partnership with RVAC Medicines.
European Pharmaceutical Review
MAY 18, 2023
Hepcludex ® (bulevirtide) is the first medicine to be conditionally licensed for chronic hepatitis delta virus (HDV) infection in Great Britain” The National Institute for Health and Care Excellence (NICE) has recommended NHS use of Hepcludex ® (bulevirtide).

Pharmaceutical Technology
JUNE 1, 2023
NICE medicines evaluation director Helen Knight said: “Each year, the lives of millions of people in England are blighted by migraine attacks. They can be extremely debilitating and can significantly affect a person’s quality of life. “In

PharmaTimes
DECEMBER 18, 2023
David Lowe, professor of health and innovation, University of Glasgow and emergency medicine consultation, NHSGGC, said: “If we can spot cancer earlier… we can improve time [for] further imaging, and subsequent treatment… [which] will help orchestrate benefits for the whole patient care pathway.”

European Pharmaceutical Review
NOVEMBER 27, 2023
According to the European Medicines Agency (EMA) and US Food and Drug Administration (FDA) , Kanuma is approved as an LAL-D treatment in countries such as the US, EU, Japan, Canada and other countries. Evidence of successful long-term management of the disease was reported in a case study published last year in the Canadian Liver Journal.

European Pharmaceutical Review
MARCH 17, 2023
The Medicines and Healthcare products Regulatory Agency (MHRA) has granted marketing authorisation for VYVGART ® (efgartigimod alfa-fcab). It is the first neonatal Fc receptor (FcRn) blocker to be approved in the UK for adults with generalised myasthenia gravis (gMG) who are anti-acetylcholine receptor (AChR) antibody positive.

European Pharmaceutical Review
NOVEMBER 14, 2023
to continue expanding its business of “helping innovators manufacture much needed genetic medicines” Miller added that the acquisition will help to advance its mission into the next global stage of development to expand its capabilities.
Pharmaceutical Technology
NOVEMBER 20, 2022
This news came as many organisations and patient advocacy groups have been campaigning for the adoption of some of the rejected drugs for several months, amidst concerns about access to medicines in the UK. At the time Amanda Pritchard, chief executive of NHS England, doubled down on the issue of medicine access. “If

European Pharmaceutical Review
AUGUST 25, 2022
For both the Innovative Medicines and Sandoz businesses, the spin-off would enable enhanced focus and the ability to pursue independent growth strategies. Meanwhile, Novartis aims to become a focused innovative medicines company with a stronger financial profile and improved return on capital. Additional transaction details.

MedCity News
NOVEMBER 7, 2022
Just 29% of employers and 34% of payers and benefits consultants said that virtual care will drastically change the way healthcare is provided and outcomes are achieved.

European Pharmaceutical Review
JANUARY 13, 2023
According to the European Pharmacopoeia Commission, the approach was defined based on: Comments received during the last round of public consultation in Pharmeuropa. Recent feedback from Heads of Medicines Agency (HMA), and European Medicines Agency (EMA), groups and from the national competent authorities of non-EU Ph.

European Pharmaceutical Review
OCTOBER 12, 2023
A new scheme from the Medicines and Healthcare products Regulatory Agency (MHRA) is set to cut the approval time for the lowest-risk clinical trials in the UK by over half. The new Notification Scheme is based on that outlined in the MHRA’s clinical trials consultation, which was endorsed by 74 percent of those who responded.

Impetus Digital
SEPTEMBER 22, 2022
The reality is that about 3% of healthcare encounters or healthcare consultations in 2019 took place through telehealth/telemedicine. And so overnight, healthcare consultations became remote. People were doing telehealth consults on Zoom. You can have a telemedicine consult like this, and you can have a conversation.

European Pharmaceutical Review
DECEMBER 20, 2023
The proposed changes to EU pharmaceutical legislation broadly aim to: Support innovation and boost attractiveness of EU market Ensure timely and equitable access to medicines for patients across the EU Address other ongoing issues, including antimicrobial resistance and environmental impact of medicines. analysis (Source: L.E.K)

European Pharmaceutical Review
FEBRUARY 3, 2023
A key consultation by the Department of Health and Social Care (DHSC) is underway on plans to raise the statutory revenue clawback rate paid by companies subject to the Statutory Scheme for branded medicines from 24.4 Between 2019 and 2023, there will have been a 12 percent real terms decline in medicines spending.
pharmaphorum
JANUARY 24, 2023
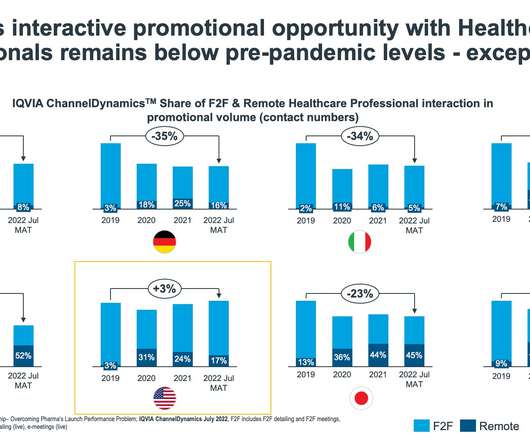
Given the funding environment for innovative medicines that is faced by all pharma, large or small, once assets are approved, however, this 2023 reset seems a necessary response. Medicines manufacturing is energy intensive. Medicines distribution and supply is also an energy intensive and regulated business.

European Pharmaceutical Review
FEBRUARY 2, 2023
The Medicines and Healthcare products Regulatory Agency (MHRA) has approved RINVOQ ® ▼ (upadacitinib), the first oral advanced therapy for adults with moderately to severely active Crohn’s disease (CD). This medicine is subject to additional monitoring.

pharmaphorum
NOVEMBER 27, 2022
The approval comes after presentation of data from the ARASENS phase 3 clinical trial at the 2022 ASCO GU Cancers Symposium in February 2022, together with simultaneous publication of the results in The New England Journal of Medicine. reduced risk of death.
European Pharmaceutical Review
AUGUST 17, 2022
Results from the Phase III VISION study have shown the significance of this precision medicine for patients with advanced prostate cancer and it is encouraging to see such innovations being recognised by the MHRA with this licensing authorisation.”. This new approach is very welcome.”.
European Pharmaceutical Review
NOVEMBER 2, 2022
The Phase I trial, published in Science Translational Medicine , involved building and applying a new generation of universal genome-edited T cells, a better solution to current chimeric antigen receptors (CAR) T- cell therapies which rely on collecting and engineering a patient’s own cells, an expensive and lengthy process.

PM360
MAY 10, 2024
Zara Baker, Chief Digital Innovation Officer Before joining VUE Health, Zara advised several pre-commercial biotechnology startups and healthcare consulting agencies in a fractional Chief Digital Officer (CDO) capacity. VUE Health is also certified as a Women’s Business Enterprise (WBE) by WBENC.

pharmaphorum
JANUARY 16, 2023
Two top pharma companies have exited the UK’s voluntary medicines pricing agreement in protest at what the industry has said is a “punitive” system of revenue clawbacks, casting doubt on the future of the scheme in its present form.

Pharma Marketing Network
JUNE 23, 2021
He has worked within life sciences organizations, leading publishers, consultancies, agencies, digital health, and technology solutions support our journeys through illness and to wellness. He often speaks and writes about his passion, EaaM – Experience as a Medicine.

MedCity News
JULY 27, 2023
However, one-size-fits-all solutions have disadvantages in medicine today. Current applications Today, there is an escalated appetite to address emerging procedures because they offer an opportunity for device makers – and physicians like me who are interested in cutting-edge technology – to influence new areas of medicine.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content