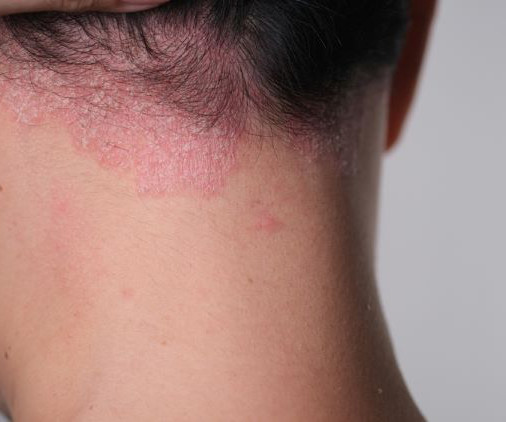
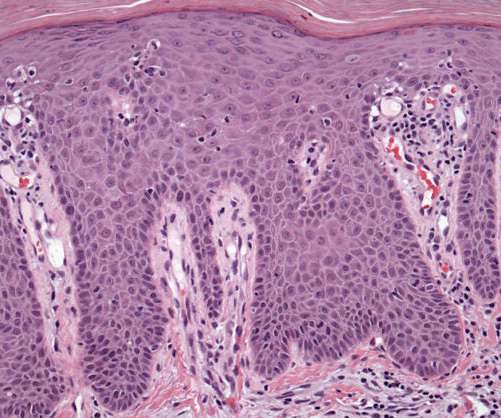
Stagnant sales for Dermavant's Vtama cast doubt on its blockbuster potential
Fierce Pharma
NOVEMBER 14, 2023
In the market, however, Vtama is struggling to catch on. As a result, Leerink Partners has slashed its sales projections for the nonsteroidal topical treatment.



































Let's personalize your content