Roche, Exelixis' Tecentriq-Cabometyx combo rebounds with partial win in previously treated prostate cancer
Fierce Pharma
AUGUST 21, 2023
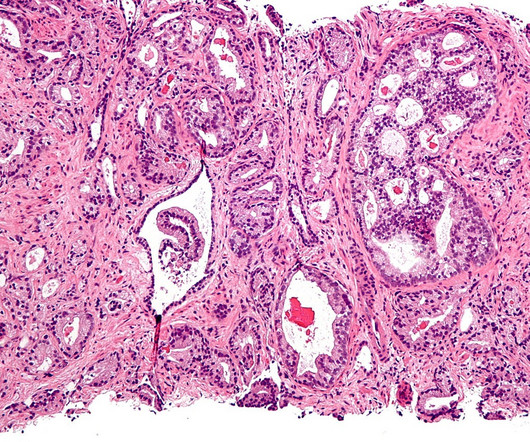
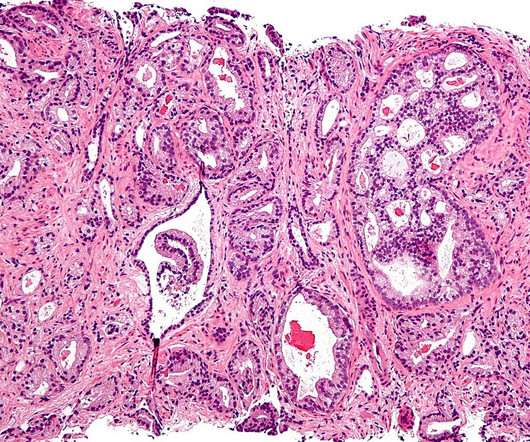
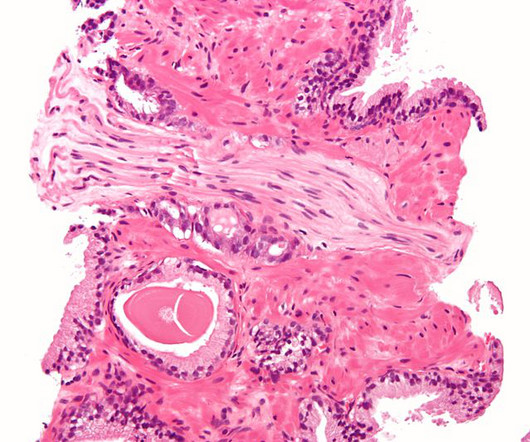
After a losing streak in the clinic, Roche and Exelixis’ combo of Tecentriq and Cabometyx has delivered a trial win in tough-to-treat prostate cancer.































Let's personalize your content