Lisata clinches rare disease status for osteosarcoma drug
Pharmaceutical Technology
MARCH 22, 2024
Lisata Therapeutics announced a rare pediatric drug designation for LSTA1, which is being studied in several early to mid-stage clinical studies.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

 clinical pediatrics
clinical pediatrics 
Pharmaceutical Technology
MARCH 22, 2024
Lisata Therapeutics announced a rare pediatric drug designation for LSTA1, which is being studied in several early to mid-stage clinical studies.
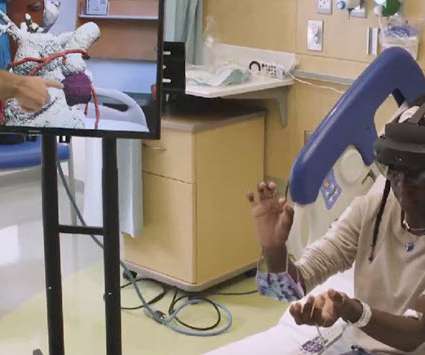
MedCity News
JUNE 27, 2022
A pediatric health system enlisting mixed reality and 3D printing technology illustrates how clinical collaboration and patient education can improve patient outcomes for complex procedures.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

MedCity News
AUGUST 12, 2022
Through the partnership, BCBSM members will have access to Maven Clinic’s app, which provides 24/7 virtual support. They can choose three program types: family building, maternity or parenting and pediatrics.
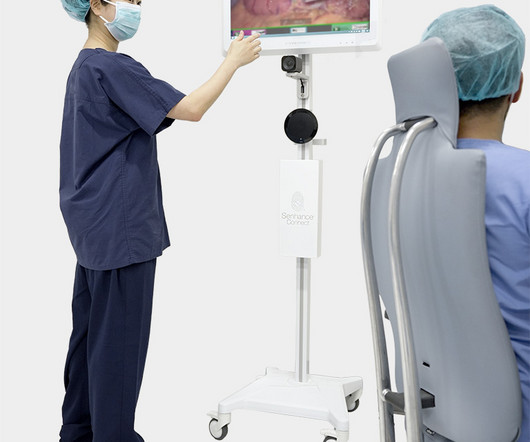
Legacy MEDSearch
MARCH 20, 2023
(NYSE American: ASXC), a medical device company that is digitizing the interface between the surgeon and the patient to pioneer a new era of Performance-Guided Surgery (PGS), announced that it has received 510(k) clearance from the FDA for an expanded indication to treat pediatric patients with the Senhance® System. Europe, and Japan.

Nixon Gwilt Law
SEPTEMBER 7, 2023
If you’re a founder or executive at a digital health company focused on pediatrics, you’re well aware that building tools to support our youngest generation’s health comes with unique advantages and challenges. Here are the critical legal issues pediatric digital health companies need to be thinking about today.
Pharmaceutical Technology
FEBRUARY 8, 2024
NiKogene is under clinical development by Kian Immune Cell and currently in Phase I for Pediatric Diffuse Intrinsic Pontine Glioma.
Pharmacy Times
AUGUST 4, 2022
Electronic symptom self-measuring provided early detection of toxic effects and helped anticipate necessary medical interventions for pediatric patients with cancer.
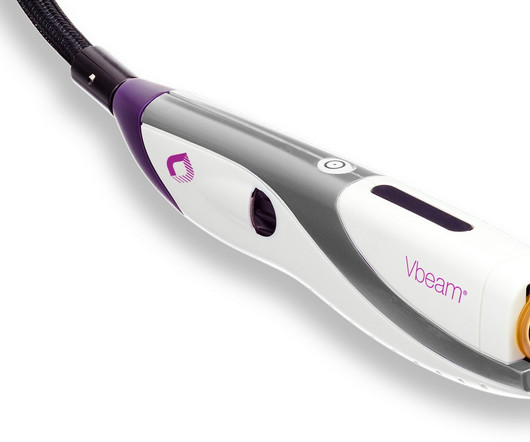
Legacy MEDSearch
JUNE 28, 2023
1 Early treatment of PWS during childhood leads to the best clinical response. 1 Early treatment of PWS during childhood leads to the best clinical response. The Vbeam 595 nm PDL is world recognized as the gold standard treatment for PWS 4,5 and the primary choice for hemangioma treatment in pediatric patients. Geronemus, M.D.,

Pharmaceutical Technology
SEPTEMBER 22, 2022
Avrobio has received rare pediatric disease designation from the US Food and Drug Administration (FDA) for its investigational gene therapy, AVR-RD-04, designed to treat cystinosis. The FDA's rare pediatric disease designation and Voucher Program aid the development of new drugs and biologics to prevent and treat rare paediatric ailments.

Medgadget
NOVEMBER 15, 2022
SpineX , a medtech company based in California, has developed the Spinal Cord Innovation in Pediatrics (SCiP) device, a non-invasive spinal cord neuromodulation technology that is intended to treat children with cerebral palsy. The children received two interventions a week during the study.
European Pharmaceutical Review
MARCH 4, 2024
Importance of the new data for the Novartis gene therapy This data is “the first Zolgensma open-label clinical study to include older and heavier, as well as previously treated patients,” according to Dr Sandra Reyna, Chief Scientific Advisor and Head of Global Medical Engagement for SMA at Novartis.

PM360
FEBRUARY 12, 2024
More than 70% of rare diseases are genetic in nature with nearly half of them impacting pediatric patients. Since rates in children are generally low, every pediatric cancer is deemed rare. Nearly 80% of clinical trials fail to finish on schedule with 20% delayed over six months or more.

Pharmacy Times
JUNE 16, 2023
Imposing the FDA on-label/off-label framework on administratively defined "children" resulted in a regulatory demand for pediatric studies that had no basis in clinical medicine, with the exception of the small group of preterm newborns.

Pharmacy Times
MAY 31, 2023
Pediatric drug development, which has a complex background and history, has fundamental catches based around how children are defined in the context of clinical studies.

Legacy MEDSearch
MAY 10, 2023
The ANNE® One platform is now cleared as a clinical-grade continuous monitoring solution for neonates, infants, and adults. “We see the ANNE® One system to be broadly applicable across the entire clinical care continuum from pediatrics to adults,” added JooHee Lee, Senior Product Manager and cofounder of Sibel Health.
Pharmaceutical Technology
MAY 17, 2023
TAK-755 is the first and only treatment in clinical development that provides targeted replacement of ADAMTS13, addressing the underlying cause of the disease. “We The Phase III trial assessed TAK-755’s clinical benefit across several clinically relevant endpoints, against plasma-based therapies, in a randomised cross-over study.
European Pharmaceutical Review
APRIL 3, 2023
These results have led to a major amendment in a European Phase III paediatric clinical trial. The oncology treatment showed clinical activity across patients of all ages with the three neuroblastoma-specific hotspot ALK mutations. The data showed 63 percent of patients responded to the combined treatment.

Pharmaceutical Technology
JUNE 5, 2023
Kid EDV is under clinical development by ImmunityBio and currently in Phase I for Pediatric Diffuse Intrinsic Pontine Glioma. According to GlobalData, Phase I drugs for Pediatric Diffuse Intrinsic Pontine Glioma does not have sufficient historical data to build an indication benchmark PTSR for Phase I.
Pharmaceutical Technology
JANUARY 17, 2023
Orphagen Pharmaceuticals has received a rare pediatric disease designation (RPDD) for OR-449 from the US Food and Drug Administration (FDA) to treat paediatric adrenocortical carcinoma (ACC). The post Orphagen’s ACC therapy receives FDA rare pediatric disease status appeared first on Pharmaceutical Technology.
European Pharmaceutical Review
AUGUST 31, 2023
Based on new data from the Novartis Phase I/II clinical trial , which used the first therapy to target a new genetic area and use cryopreserved stem cells, trial participants reported a decrease in vaso-occlusive events. This is where sickled red blood cells accumulate and cause a blockage.

pharmaphorum
NOVEMBER 11, 2022
Leading with practical insights from the best in the industry, the 4th Annual Inflammasome Therapeutics Summit is returning in person on November 29 – December 1 to Boston to equip you with the knowledge and connections to propel the next frontier of inflammasome therapeutics from pipeline to clinical and commercial reality.

European Pharmaceutical Review
SEPTEMBER 8, 2022
Lurie Children’s Hospital of Chicago and Professor of Pediatrics at Northwestern University Feinberg School of Medicine, both US. Regulators call for clinical research to include pregnant and breastfeeding women… The post Dolutegravir shows promise in pregnancy appeared first on European Pharmaceutical Review.
Pharmaceutical Technology
FEBRUARY 21, 2023
The findings showed that 100% of patients had quick and sustained normalisation of serum albumin (a disease biomarker) and improvement or no worsening of clinical symptoms at 24 weeks. It was also granted Orphan Drug Designation during the same time.

Pharma Leaders
APRIL 14, 2023
The gene therapy candidate is being evaluated in a phase 1/2 clinical trial, in which six patients ages four to 11 years are expected to enroll for a one-time intravenous dose of the medication. The agency previously granted RGX-202 Orphan Drug and Rare Pediatric Disease designations. Related Topics

European Pharmaceutical Review
JULY 25, 2023
Additionally, in the 0.3mg/kg group, 55 percent of patients experienced a clinically meaningful improvement in symptoms. more effective treatment options for this significant complication are desperately needed,” urged Dr Carrie Kitko, Medical Director of the Pediatric Stem Cell Transplant Program at the Vanderbilt-Ingram Cancer Center.

pharmaphorum
JULY 5, 2022
With more than 500 dermatology clinical trials currently underway, it is often heard that there is a ‘’competition for participants’’. Instead, atopic dermatitis clinical trial organisers often simply only reach out to a limited patient population and fail to reach the complete, diverse, patient population of atopic dermatitis.

Pharmaceutical Technology
JUNE 5, 2023
Pralsetinib is under clinical development by F. Attributes of the drug, company and its clinical trials play a fundamental role in drug-specific PTSR and likelihood of approval. Hoffmann-La Roche and currently in Phase II for Pancreatic Cancer. Pralsetinib overview Pralsetinib (Gavreto) is an anticancer agent.

Pharmaceutical Technology
FEBRUARY 9, 2023
Ibutamoren mesylate is under clinical development by Lumos Pharma and currently in Phase I for Turner Syndrome. Attributes of the drug, company and its clinical trials play a fundamental role in drug-specific PTSR and likelihood of approval.
Pharmaceutical Technology
OCTOBER 6, 2022
Additionally, GBT’s pipeline of preclinical and clinical investigational assets for SCD includes GBT021601 (GBT601) and inclaclumab. The FDA has granted Orphan Drug and Rare Pediatric Disease designations for these two assets.

Pharmacy Times
AUGUST 17, 2022
Zynteglo was approved for the treatment of adult and pediatric patients with beta-thalassemia who require regular red blood cell transfusions after safety and efficacy were demonstrated in clinical trials.

Pharmaceutical Technology
FEBRUARY 9, 2023
Ibutamoren mesylate is under clinical development by Lumos Pharma and currently in Phase II for Growth Hormone Deficiency. Attributes of the drug, company and its clinical trials play a fundamental role in drug-specific PTSR and likelihood of approval.

European Pharmaceutical Review
FEBRUARY 8, 2024
Vertex Pharmaceuticals’ once-daily small molecule vanzacaftor/tezacaftor/deutivacaftor (vanza triple) for cystic fibrosis (CF) has gleaned positive results in Phase III trials.

Pharmaceutical Technology
SEPTEMBER 16, 2022
Currently in the clinical stage, the antibody is being developed as a stem cell transplant conditioning agent. In an ongoing multicentre clinical trial, JSP191 has so far been analysed in 14 patients with SCID. It aids in creating a vacant space for the donor or gene-corrected transplanted stem cells to be engrafted. .
Legacy MEDSearch
JULY 11, 2023
The functionality and performance of the Neoasis ® were highlighted in a recent presentation at the Pediatric Academic Society conference. “In In previously published clinical studies, this level of noise attenuation resulted in improved sleep hygiene and rate of growth.” Invictus Medical is a privately held company.

Referral MD
NOVEMBER 16, 2023
With eConsults, ReferralMD improves the patient experience, reduces costs, and expands the scope of primary care by providing crucial clinical guidance from specialists. Primary care providers can confer with specialists through eConsults before making referrals for face-to-face care. To learn more about ReferralMD, please visit [link].

Pharmaceutical Technology
NOVEMBER 9, 2022
On November 2, the Institute for Clinical and Economic Review (ICER) released its updated evidence aimed at measuring the clinical effectiveness and cost of the two haemophilia gene therapies. Known by the brand name Roctavian, BioMarin’s haemophilia A therapy valoctocogene roxaparvovec could be fairly priced in the range of $1.95–1.96
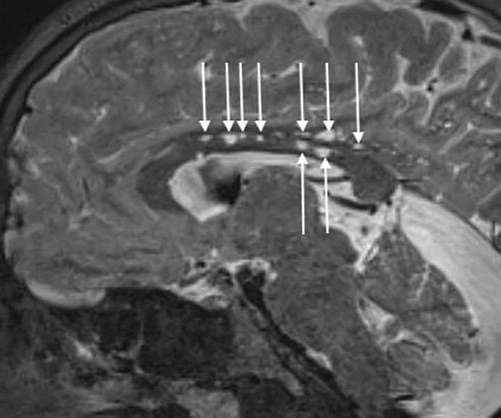
Pharmaceutical Technology
JULY 14, 2022
AVR-RD-05 had previously obtained rare pediatric disease designation from the FDA. Next year, Avrobio is expected to initiate a collaborator-sponsored Phase I/II clinical trial for Hunter syndrome in partnership with the University of Manchester, UK.

Clarify Health
MAY 18, 2023
Last September, CHI released “The Kids Are Not Alright: Pediatric Mental Health Care Utilization from 2016-2021,” which looked at mental health utilization among children with a diagnosed mental illness.

World of DTC Marketing
AUGUST 13, 2021
A TELLING SPIKE: Dr. Brynn Marks, a pediatric endocrinologist at Children’s National Hospital in Washington, D.C., Brynn Marks, a pediatric endocrinologist at Children’s National Hospital in Washington, D.C., …At Children’s National Hospital in Washington, D.C.,
Legacy MEDSearch
NOVEMBER 28, 2022
Based on customer feedback, the company designed the BodyTom 64 to enhance the user experience and improve clinical workflows through revisions to both the software and the data acquisition system (DAS). We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search.
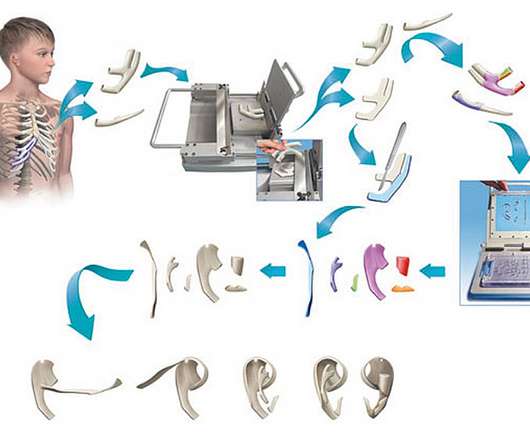
Medgadget
OCTOBER 11, 2022
At Johns Hopkins, clinical researchers have developed a set of surgical tools that allow them to more easily create a replacement ear for those born with malformed or missing ears. This is difficult – surgeons are clearly very skilled with their hands, but artistic skills, such as sculpting, often are a little outside their comfort zone.

Pharmaceutical Technology
FEBRUARY 19, 2023
STRO-002 is under clinical development by Sutro Biopharma and currently in Phase I for Refractory Acute Myeloid Leukemia. Attributes of the drug, company and its clinical trials play a fundamental role in drug-specific PTSR and likelihood of approval. It is administered through intravenous route.

Pharmaceutical Technology
MARCH 2, 2023
Rituximab biosimilar is under clinical development by Dr. Reddy’s Laboratories and currently in Phase III for Rheumatoid Arthritis. Attributes of the drug, company and its clinical trials play a fundamental role in drug-specific PTSR and likelihood of approval. The company also develops and markets generic biosimilar products.

Legacy MEDSearch
MARCH 22, 2023
Designates FibroScan as a non-invasive aid for the clinical management, diagnosis, and monitoring of adult and pediatric patients with confirmed or suspected liver disease as part of an overall assessment of the liver. ” Age has been removed as the first selection probe and exam-type step.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?

Let's personalize your content