First immunotherapy recommended for advanced cervical cancer
European Pharmaceutical Review
MARCH 29, 2023
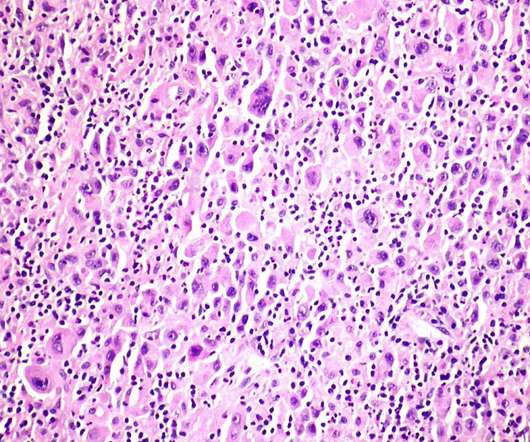
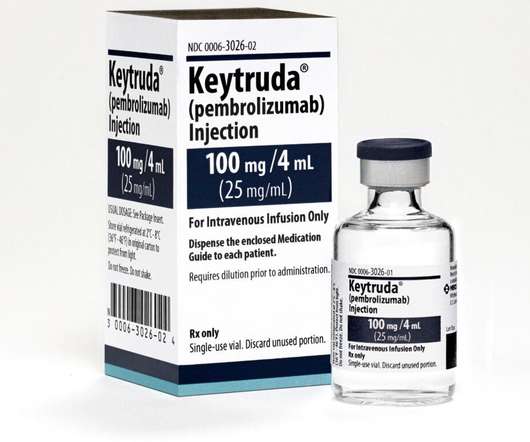
Pembrolizumab (Keytruda) has been recommended in National Institute for Health and Care Excellence (NICE) final draft guidance for advanced cervical cancer. Clinical trial evidence shows that the treatment helps delay worsening of the disease , compared to standard care.


































Let's personalize your content