FDA Reconsideration Leads to Approval of Takeda Drug for Rare Esophagus Disorder
MedCity News
FEBRUARY 12, 2024
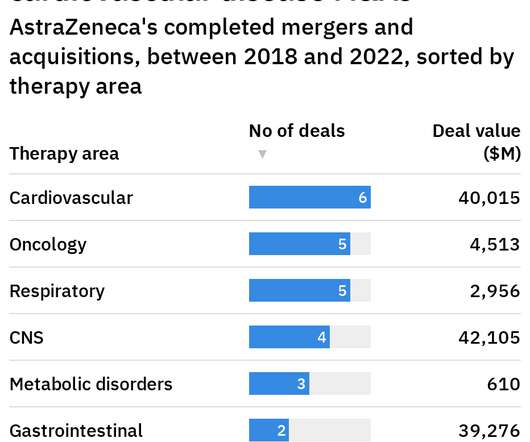
FDA approval of Takeda Pharmaceutical drug Eohilia introduces a new therapeutic option for patients with eosinophilic esophagitis, inflammation of the esophagus that causes swallowing difficulty. It will compete against Dupixent, a Sanofi and Regeneron Pharmaceuticals drug already approved for this disorder.







































Let's personalize your content