High risk, high reward – the future of CAR-T therapy in CLL
Pharmaceutical Technology
OCTOBER 5, 2022
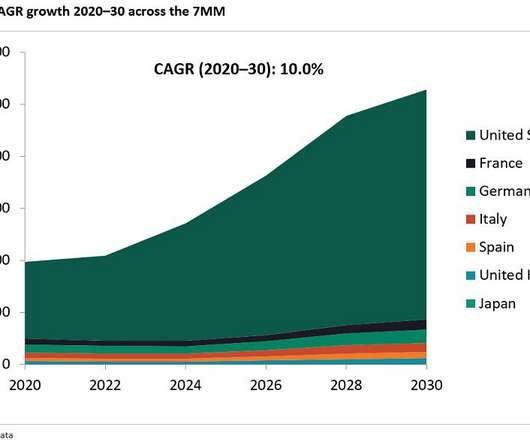
According to GlobalData’s analyst consensus forecast, Yescarta is expected to reach peak sales of $1.8bn in 2028, and Kymriah is expected to reach sales of $686m in the same year.
















Let's personalize your content