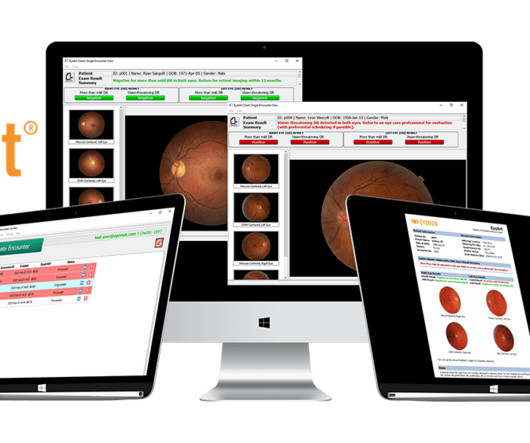
iHealthScreen Inc. Announces FDA 510K Submission for iPredict™ AMD tool
Legacy MEDSearch
MAY 24, 2023
iHealthScreen conducted a prospective trial in the general population to prospectively assess the product’s accuracy, sensitivity and specificity. These prospective study results were initially presented at the Annual Meeting of the American Academy of Ophthalmology (AAO) and selected for Panel Discussion as outstanding work.





















Let's personalize your content