AstraZeneca and MSD’s Lynparza combo bags FDA approval for prostate cancer
Pharmaceutical Technology
JUNE 1, 2023
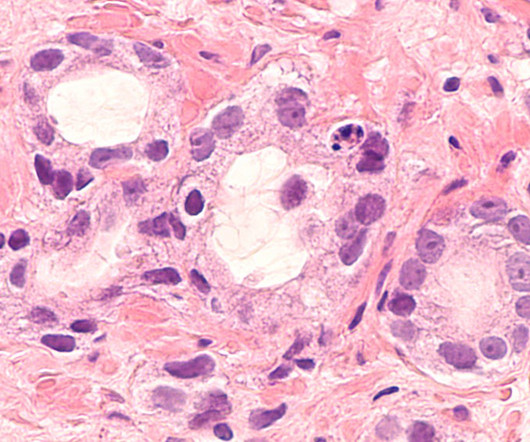
The US Food and Drug Administration (FDA) has granted a combination of AstraZeneca and MSD ’s Lynparza (olaparib), with standard therapies for treating BRCA-mutated (BRCAm) metastatic castration-resistant prostate cancer (mCRPC). Safety and tolerability were in line with that observed in prior trials and the known profiles of the medicines.



































Let's personalize your content