EVERSANA and Amazon Web Services Introduce Transformative Medical & Regulatory Review AI Solution
PM360
NOVEMBER 27, 2023
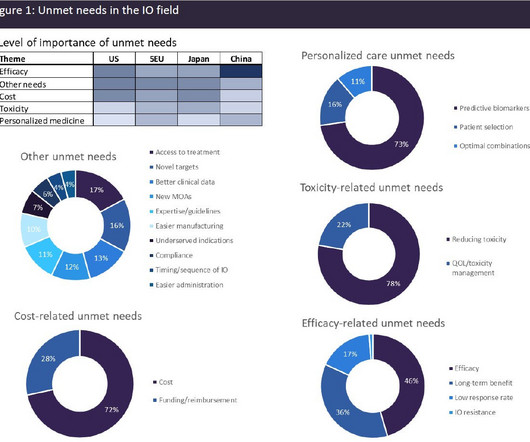
The first solution, developed by EVERSANA leveraging AWS and AWS Partner TensorIoT , addresses a time-consuming and error-prone process in the industry—medical and regulatory content approvals. As we came together with AWS, it became clear we could make a real impact in the medical and regulatory review process for branded materials.






















Let's personalize your content