bluebird bio wins back-to-back landmark FDA approvals for first-in-class gene therapies
Pharmaceutical Technology
SEPTEMBER 30, 2022
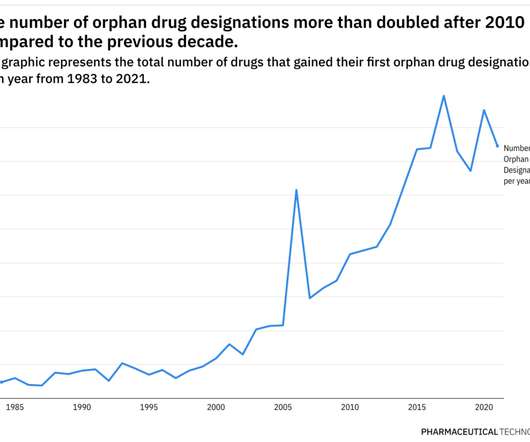
After several setbacks, bluebird bio bounces back with two major FDA gene therapy approvals. Last month, Zynteglo (betibeglogene autotemcel), or beti-cel, was approved as a one-time potentially curative gene therapy for patients with beta-thalassaemia who require regular blood transfusions.















Let's personalize your content