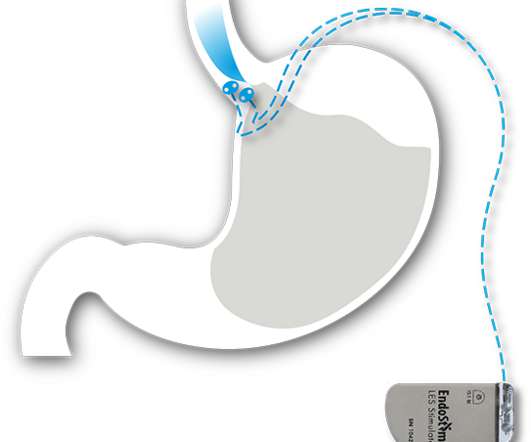
EndoStim Receives FDA Breakthrough Device Designation for the EndoStim System for the Treatment of Drug Refractory GERD
Legacy MEDSearch
OCTOBER 25, 2022
EndoStim , a medical device company developing and commercializing a first-in-class implantable neurostimulation treatment for drug refractory gastroesophageal reflux disease (GERD), announced that the U.S. Food and Drug Administration (FDA) granted Breakthrough Device Designation for the Company’s EndoStim System.





































Let's personalize your content