Drug safety driving pyrogen testing market expansion
European Pharmaceutical Review
MARCH 27, 2024
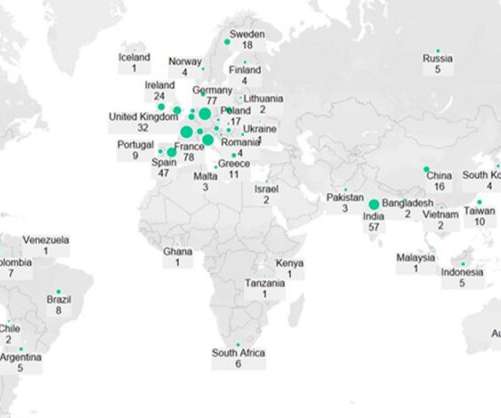
Additionally, the authors highlighted that a greater number of pharmaceutical and biotechnology contract manufacturing organisations (CMOs) has broadened the opportunities for providers of pyrogen testing services. This was attributed to their “highly sensitive and specific” testing capabilities.



































Let's personalize your content