Injectable drug delivery market to value $1139.4b by 2029
European Pharmaceutical Review
MAY 7, 2024
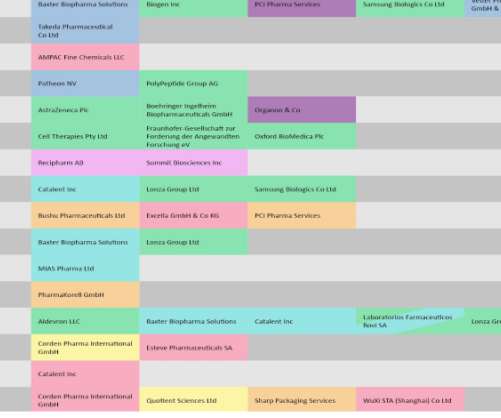
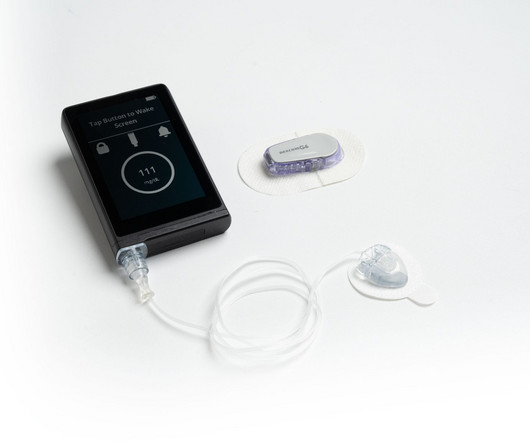
A new report by MarketsandMarkets has predicted that the injectable drug delivery market will reach an 8.6 Injectable drugs are adopted widely as treatments for chronic infections such as HIV/AIDS and tuberculosis (TB). This has resulted in more access to injectable drugs. Teva Pharmaceuticals Industries Ltd.,


































Let's personalize your content