Challenges in the Medical Sales Industry
MedReps
SEPTEMBER 19, 2022
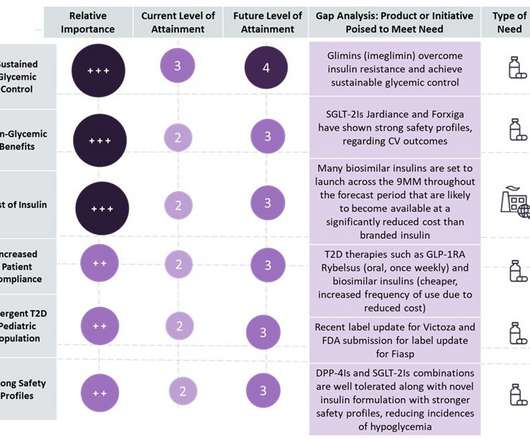
There’s no shortage of challenges for those new to pharma, medical sales and even veterans. Often we’re lucky if we even get to see our physicians, let alone overcome their objections regarding insurance coverage and competitive claims. Below are seven common challenges that pharmaceutical and other medical sales reps face.






























Let's personalize your content