Pharma’s salesforce is suffering — what does version 2.0 look like?
PharmaVoice
MARCH 28, 2023
Ahead of turbulent market conditions, EY analyst Arda Ural encourages companies to rethink their sales strategy.

PharmaVoice
MARCH 28, 2023
Ahead of turbulent market conditions, EY analyst Arda Ural encourages companies to rethink their sales strategy.

Pharmaceutical Commerce
MARCH 30, 2023
With the rapid rise of the specialty drug market, manufacturers are increasingly relying on new advances used by patient hubs to boost their levels of patient engagement.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

MedCity News
MARCH 29, 2023
Demand for alternative drug delivery methods has the potential to reshape therapeutic markets and improve clinical outcomes. New innovations, especially oral alternatives, are poised to drive broad changes across the entire sector.

Fierce Pharma
MARCH 30, 2023
As deadly pathogen spreads, GSK throws a lifeline to Scynexis with $90M deal to market antifungal aliu Thu, 03/30/2023 - 10:39
Pharmaceutical Technology
MARCH 31, 2023
The European Medicines Agency (EMA) has recommended HIPRA’s Covid-19 vaccine , Bimervax, as a Covid-19 booster. Available to people ages 16 years and above who have been vaccinated with a Covid-19 mRNA vaccine, EMA’s Human Medicines Committee concluded the vaccine is ready for marketing authorization in the EU, on 30 March. Bimervax is a recombinant protein subunit vaccine, marketed by the Girona, Spain-based Hipra.

European Pharmaceutical Review
MARCH 27, 2023
A report has predicted the rapid microbiology testing market size is estimated to grow at a compound annual growth rate (CAGR) of 9.31 percent between 2022 and 2027. According to the data, the market is forecasted to increase by $2,487.91 million. The research includes historic market data from 2017 to 2021, using 2022 as the base year. It covers various testing methods such as growth-based, nucleic-acid-based and viability-based.
Pharma Rep Focus brings together the best content for pharma rep professionals from the widest variety of industry thought leaders.

Fierce Pharma
MARCH 27, 2023
BioNTech surprises analysts with low revenue guidance as COVID-19 vaccine demand wavers fkansteiner Mon, 03/27/2023 - 09:35

Pharmaceutical Technology
MARCH 28, 2023
Following on from its Covid-19 vaccine programmes, BioNTech has set its sights on a range of infectious diseases for vaccine development. In its FY 2022 report, BioNTech has identified herpes simplex virus (HSV), malaria, and shingles as disease targets. The company saw major successes with its Covid-19 vaccine, developed in collaboration with Pfizer.

European Pharmaceutical Review
MARCH 31, 2023
The Medicines Patent Pool (MPP) has signed sublicence agreements with three generics manufacturers to produce generic versions of cabotegravir long-acting (LA) injectable for human immunodeficiency virus (HIV) pre-exposure prophylaxis (PrEP). First sublicence agreement signed by MPP for a long-acting medicine Through the sublicences, the selected generic manufacturers will be able to develop, manufacture, and supply generic versions of cabotegravir LA for PrEP, in 90 countries, subject to requir

MedCity News
MARCH 30, 2023
The recent growth in RPM has demonstrated its importance and potential to reduce or eliminate barriers and improve access to care for patients. By leveraging RPM and other digital health solutions as an important part of a multi-prong approach to care delivery, a future where all patients have equal access to high-quality healthcare becomes a closer reality.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Fierce Pharma
MARCH 27, 2023
Novo's high-dose Rybelsus win tees up regulatory filings—and new rivalries esagonowsky Mon, 03/27/2023 - 09:19

Pharmaceutical Technology
MARCH 31, 2023
Moderna has finalised an agreement with the government of the Republic of Kenya to establish an mRNA manufacturing facility in the country. The company will construct the new advanced mRNA facility, which is claimed to be Moderna’s first mRNA manufacturing facility in Africa and is expected to have the capacity to produce up to 500 million vaccine doses annually.
Medgadget
MARCH 27, 2023
Researchers at the Georgia Institute of Technology have developed a nanotechnological solution for lymphedema, a failure of the lymphatic system that results in uncomfortable and irreversible fluid retention. Previous research efforts have focused on trying to grow new lymphatic vessels, but these researchers have taken a different approach, and instead engineered a drug delivery technology that can directly target sluggish lymphatic vessels and kickstart their pumping action.

MedCity News
MARCH 29, 2023
The financial outlook for the digital health sector is less than ideal, characterized by lower funding amounts, shrinking valuations, dwindling profitability and the collapse of Silicon Valley Bank. During a recent conference panel, four healthcare VCs discussed their reactions to this. They explained why the market has switched its focus from growth to value, as well gave advice to startups about what they need to know in this changing environment.

Advertiser: ZoomInfo
Marketing technology is essential for B2B marketers to stay competitive in a rapidly changing digital landscape — and with 53% of marketers experiencing legacy technology issues and limitations, they’re researching innovations to expand and refine their technology stacks. To help practitioners keep up with the rapidly evolving martech landscape, this special report will discuss: How practitioners are integrating technologies and systems to encourage information-sharing between departments and pr

Fierce Pharma
MARCH 27, 2023
Duo no more: After M&A spree, Pfizer parts ways with Merck KGaA on Bavencio zbecker Mon, 03/27/2023 - 17:42

Pharmaceutical Technology
MARCH 29, 2023
Viking Therapeutics has announced the start of a Phase I clinical study to evaluate its dual glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) receptor agonist VK2735. The trial, which is investigating healthy adults with a minimum body mass index of 30 kg/m2, will see VK2735 administered as an oral tablet once daily for 28 days.

PharmaVoice
MARCH 28, 2023
The Supreme Court will now decide whether Amgen can protect Repatha as a whole class of drugs or whether Sanofi’s rival product Praluent can retain a place in the market.

MedCity News
MARCH 26, 2023
To build remote patient monitoring technology for ready adoption, repetitive data streams must be avoided. To be successful in clinical adoption, we offer technology companies the following three recommendations.

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.

Fierce Pharma
MARCH 27, 2023
With FDA nod for a Novartis castoff, Pharming is set to market a second rare disease drug kdunleavy Mon, 03/27/2023 - 10:40
Pharmaceutical Technology
MARCH 27, 2023
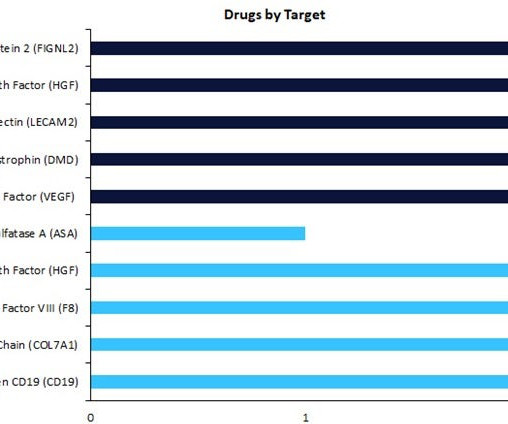
Regenerative medicines in early-stage development (preclinical, discovery, or investigational new drug [IND]/ clinical trial application [CTA] filed status) have seen a change in drug targets compared to therapies in late-stage development (Phase II to pre-registration stage). According to GlobalData’s Drugs database, early-stage therapies are focused on vascular endothelial growth factor (VEGF) as the top drug target, with three drugs currently in development.
Progressive Medical
MARCH 28, 2023
Progressive Medical Awarded National Agreement with Premier Inc. PMI is pleased to announce that it has been awarded a […] The post Progressive Medical, Inc. Awarded Drug Reconstitution and Transfer Devices Agreement with Premier, Inc. | Yukon appeared first on Progressive Medical, Inc.

MedCity News
MARCH 30, 2023
The potential of ChatGPT is exciting. Radiologists have come a long way on the AI journey. What started as a fear from some that AI would replace us, has evolved to a more nuanced understanding that AI’s greatest contribution in medical imaging is to make us better radiologists.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Fierce Pharma
MARCH 30, 2023
Pfizer, BMS' Eliquis tops list of drugs destined for Medicare price negotiations in 2026: Moody's kdunleavy Thu, 03/30/2023 - 07:28
Pharmaceutical Technology
MARCH 29, 2023
Regeneron Pharmaceuticals has collaborated with Sonoma Biotherapeutics to discover, develop and commercialise new regulatory T cell (Treg) therapies for autoimmune diseases. The partnership will combine the VelociSuite technologies of Regeneron with Sonoma Biotherapeutics’ pioneering approach to develop and produce gene modified Treg cell therapies.

PharmExec
MARCH 30, 2023
At Pharma USA 2023, Peter Marks, MD, PhD and director of CBER described FDA and public/private partnerships working toward scalable manufacturing approaches to address commercial viability and global access for cell and gene therapies

MedCity News
MARCH 28, 2023
While alcohol-based hand sanitizers are very useful in settings where there is an increase in the transmission of pathogens (such as hospitals), there are no additional health benefits to habitual hand sanitizing unless a person doesn’t have access to running water.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.
Fierce Pharma
MARCH 29, 2023
Boehringer Ingelheim and Eli Lilly's Jardiance gets big lift from heart failure indications kdunleavy Wed, 03/29/2023 - 10:40

Pharmaceutical Technology
MARCH 28, 2023
Vertex Pharmaceuticals has signed a new non-exclusive licensing agreement with CRISPR Therapeutics to expedite the development of its hypoimmune cell therapies to treat type 1 diabetes (T1D). Under the terms of the deal, the company will receive non-exclusive rights to CRISPR/Cas9, a gene-editing technology of CRISPR Therapeutics, for the development of potentially curative T1D cell therapies.
European Pharmaceutical Review
MARCH 29, 2023
Pembrolizumab (Keytruda) has been recommended in National Institute for Health and Care Excellence (NICE) final draft guidance for advanced cervical cancer. A promising immunotherapy “Pembrolizumab shows promise as the first effective immunotherapy [for advanced cervical cancer]” “Pembrolizumab shows promise as the first effective immunotherapy” for the condition, stated Helen Knight, Director of Medicines Evaluation at NICE.

MedCity News
MARCH 29, 2023
As many as 25 million Americans over the age of five say they speak English “less than well” — that’s nearly a tenth of the country. Contrast that with the fact that nearly one third of American hospitals don’t provide interpreters to patients who speak little English, even though it is required by federal law. Only 13 states and Washington, D.C. provide reimbursements for the cost of medical interpreters through Medicaid.

Advertiser: ZoomInfo
In times of economic uncertainty, account-based strategies are essential. According to several business analysts and practitioners, ABM is a necessity for creating more predictable revenue. Research shows that nearly three-quarters of marketers (74%) already have the resources needed to build successful ABM programs.
Let's personalize your content