White House wins in first Medicare negotiation legal ruling
pharmaphorum
FEBRUARY 13, 2024
Texas judge has thrown out the first lawsuit brought by PhRMA claiming Medicare negotiation of drug pricing is unconstitutional

pharmaphorum
FEBRUARY 13, 2024
Texas judge has thrown out the first lawsuit brought by PhRMA claiming Medicare negotiation of drug pricing is unconstitutional

Fierce Pharma
FEBRUARY 16, 2024
The T-cell therapy treatment class, which has transformed the treatment of certain blood cancers, has now reached the solid tumor field thanks to an FDA approval for a first-of-its-kind immunothera | The T-cell therapy treatment class, which has transformed the treatment of certain blood cancers, has now reached the solid tumor field thanks to an FDA approval for a first-of-its-kind immunotherapy developed by Iovance Biotherapeutics.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

MedCity News
FEBRUARY 13, 2024
Exscientia’s termination of CEO Andrew Hopkins is for cause and effective immediately. Grounds for termination include misconduct or behavior that brings Hopkins or the company into disrepute, according to his employment agreement.
Pharmaceutical Technology
FEBRUARY 12, 2024
The UK MHRA has granted approval for a variation in licence of Pfizer-BioNTech’s Comirnaty XBB.1.5 vaccine targeting Omicron variant.

PM360
FEBRUARY 12, 2024
This February we celebrate the 16th annual Rare Disease Day , established in 2008, to raise awareness of the more than 7,000 identified rare diseases worldwide. Rare Disease Day is observed annually on the “rarest” day of the year, February 28th or 29th depending on leap years. Created by The European Organization for Rare Diseases (EURORDIS) , this globally coordinated event brings together industry stakeholders to advocate for increased access to medical treatment for patients, caregivers, and

Fierce Pharma
FEBRUARY 16, 2024
People with food allergies finally have a drug that can help prevent severe outcomes—and it’s a drug that’s been on the market for two decades. | People with food allergies finally have a drug that can help prevent severe outcomes—and it’s a drug that’s been on the market for two decades. The FDA has blessed Roche and Novartis’ Xolair as the first medicine to reduce allergic reactions that can occur with accidental exposure to certain foods.
Pharma Rep Focus brings together the best content for pharma rep professionals from the widest variety of industry thought leaders.
pharmaphorum
FEBRUARY 16, 2024
CAR-T therapies can achieve remarkable efficacy in the treatment of haematological cancers, but the risk of side effects means that the cell infusions are almost always administered to inpatients under close supervision in clinics.

Pharmaceutical Technology
FEBRUARY 15, 2024
The success of recent trials treating mental health problems with psychedelic therapies has driven a rapid rise in investment in the area.

Fierce Pharma
FEBRUARY 16, 2024
Gene editing’s therapeutic application has transitioned from hypothetical to reality, marked by the recent approval of a CRISPR-based therapy for sickle cell and beta thalassemia. | This week on "The Top Line," Max Bayer from Fierce Biotech explores the future of gene editing in an interview with the CEO of Verve Therapeutics.

MedCity News
FEBRUARY 16, 2024
Offering safe affordable family-forming programs for everyone, including vulnerable or under-served employee populations, represents an opportunity for employers to build a caring culture that also will benefit the bottom line.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
Pharmaceutical Commerce
FEBRUARY 15, 2024
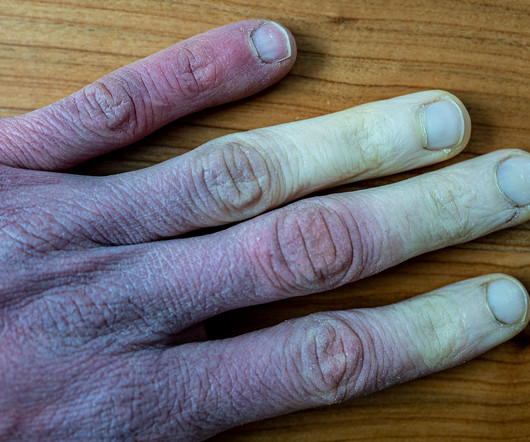
Patients with severe frostbite who were administered Aurlumyn monotherapy experienced a significantly reduced risk of the need for amputation.

pharmaphorum
FEBRUARY 13, 2024
Biogen’s Skyclarys is the first approved medicine for the inherited neurological disease Friedreich’s ataxia (FA) in the EU, after getting a green light from the European Commission.

Fierce Pharma
FEBRUARY 15, 2024
Even as Catalent moves toward a $16.5 billion sale to Novo Holdings, hundreds of the CDMO's former staffers won't be in the mix going forward. | A recent buyout announcement didn't spare Catalent staffers from layoffs after the company this week revealed it's trimmed hundreds of jobs over the last few months. The move forms part of an ongoing restructuring scheme at Catalent.

MedCity News
FEBRUARY 16, 2024
As more and more organizations integrate Generative AI with Configure, Price, Quote (CPQ) systems, they declare their belief that we can aspire to incredible advancements in human health and well-being through digital transformation.

Advertiser: ZoomInfo
Marketing technology is essential for B2B marketers to stay competitive in a rapidly changing digital landscape — and with 53% of marketers experiencing legacy technology issues and limitations, they’re researching innovations to expand and refine their technology stacks. To help practitioners keep up with the rapidly evolving martech landscape, this special report will discuss: How practitioners are integrating technologies and systems to encourage information-sharing between departments and pr
European Pharmaceutical Review
FEBRUARY 15, 2024
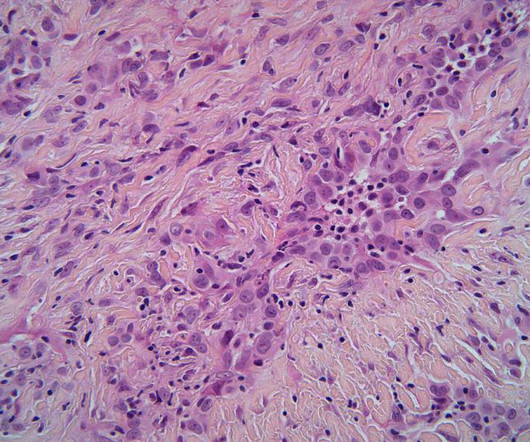
A clinical trial in malignant pleural mesothelioma (MPM) has demonstrated the first successful combination of chemotherapy with a drug targeting cancer’s metabolism developed for the asbestos-induced disease in two decades. The anti-cancer treatment combines the new drug ADI-PEG20 and traditional chemotherapy. The Phase III ATOMIC-meso trial was led by Queen Mary University of London in the UK and studied 249 patients with the disease.

pharmaphorum
FEBRUARY 16, 2024
Recent biotech financings include a $245m raise for cancer blood test company Freenome, plus big rounds for Cogent Biosciences, BioAge Labs, Latigo Biotherapeutics, ProfoundBio, and Firefly Bio

Fierce Pharma
FEBRUARY 14, 2024
FDA unleashes multiple warning letters targeting insanitary manufacturing and online sales of unapproved Mounjaro, Ozempic fkansteiner Wed, 02/14/2024 - 05:10
MedCity News
FEBRUARY 14, 2024
Ipsen’s Onivyde is now FDA approved as a first-line treatment for metastatic pancreatic cancer, triggering a milestone payment to Merrimack Pharmaceuticals, the drug’s original developer. Merrimack plans to dissolve operations but its shareholders will receive payouts from the Ipsen payment.

Speaker: Dr. Ben Locwin - Biopharmaceutical Executive & Healthcare Futurist
What will the future hold for clinical research? A recent draft from the FDA provides valuable insight. In "Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases," the FDA notes that "targeted therapies demonstrate different dose-response relationships compared to cytotoxic chemotherapy, such that doses below the Maximum Tolerated Dose (MTD) may have similar efficacy to the MTD but with fewer toxicities.

Pharmaceutical Technology
FEBRUARY 13, 2024
The MHRA kicks off phase two of the Yellow Card biobank to explore the genetic link to side effects of direct oral anticoagulants.

pharmaphorum
FEBRUARY 16, 2024
Gilead Sciences has paused enrolment in clinical trials of its CD47 drug magrolimab in solid tumours, a week after dropping it for blood cancers.
Fierce Pharma
FEBRUARY 14, 2024
Over the last month, for those watching HBO’s macabre “True Detective Night Country”—a TV series set in a harsh, sunless winter in a fictional Alaskan town—a continual theme is frostbite. | The FDA has approved the first medicine for severe frostbite. The U.S. regulator has signed off on Johnson & Johnson’s Aurlumyn (iloprost), an injected treatment to reduce the risk of amputation of the fingers or toes.

MedCity News
FEBRUARY 15, 2024
Through a new partnership, up to 12 million members of Cigna’s employer customers will gain access to HelloFresh’s meals at a discounted rate. Cigna and HelloFresh are also partnering to provide free meal kits to those battling food insecurity in local communities.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Pharmaceutical Technology
FEBRUARY 15, 2024
As the market is progressively becoming saturated by GLP-1 receptor agonists, companies are investigating alternative mechanisms of action.

PharmaTimes
FEBRUARY 14, 2024
The aggressive type of cancer accounts for 13% of all lung cancer cases worldwide

Fierce Pharma
FEBRUARY 13, 2024
While Biogen’s financials are in rough shape now, the company’s CEO, Chris Viebacher, sees reason to be optimistic about the future. | With four new drug launches rolling and the majority of the company’s losses of exclusivity in the rearview, Biogen figures it could chart continued revenue growth over the next 10 years, CEO Chris Viehbacher said Tuesday.
MedCity News
FEBRUARY 12, 2024
By leveraging AI and machine learning, researchers can analyze complex data sets, identify novel biomarkers, and provide more effective and tailored treatment options for cancer patients.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.

pharmaphorum
FEBRUARY 12, 2024
Study finds that monitoring of rheumatoid arthritis patients with an Apple Watch and iPhone and AI algorithm improves on standard practices.

European Pharmaceutical Review
FEBRUARY 15, 2024
The investment… includes state-of-the-art instrumentation to provide full CMC support to serve the growth of PODP [manufacturers]” Nelson Labs, a global leader in microbiological and analytical chemistry testing and advisory services for the medical device and pharmaceutical industries has announced its Itasca, Illinois laboratory in the US as a new Pharmaceutical Center of Excellence to serve the unique needs of parenteral and ophthalmic drug product (PODP) manufacturers.
Fierce Pharma
FEBRUARY 15, 2024
Changing a clinical trial’s statistical analysis plan on the cusp of a readout? | Changing a clinical trial’s statistical analysis plan on the cusp of a readout? That’s exactly what Alnylam just did for a closely watched study of its next-generation RNA interference therapy Amvuttra in a rare heart disease.

MedCity News
FEBRUARY 11, 2024
HHS recently published guidance outlining voluntary cybersecurity performance goals for the healthcare sector. Taylor Lehmann, a cybersecurity executive at Google Cloud, noted that “what is on HHS paper will most likely become what is in the actual final rulemaking or new regulatory requirements that become law.

Advertiser: ZoomInfo
In times of economic uncertainty, account-based strategies are essential. According to several business analysts and practitioners, ABM is a necessity for creating more predictable revenue. Research shows that nearly three-quarters of marketers (74%) already have the resources needed to build successful ABM programs.
Let's personalize your content