Biopharma staffers exhausted and susceptible to burnout, report says
Fierce Pharma
MAY 24, 2023
Biopharma staffers exhausted and susceptible to burnout, report says kdunleavy Wed, 05/24/2023 - 16:52

Fierce Pharma
MAY 24, 2023
Biopharma staffers exhausted and susceptible to burnout, report says kdunleavy Wed, 05/24/2023 - 16:52
MedCity News
MAY 24, 2023
Multiple efforts are underway to improve cell therapies for cancer. During the MedCity News INVEST conference in Chicago, a panel discussed the future of cell therapies and what the industry doing to manage the financial risks of these new treatments.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Fierce Pharma
MAY 24, 2023
After years of litigation, Gilead and Teva's HIV antitrust trial kicks off in California zbecker Wed, 05/24/2023 - 17:17
MedCity News
MAY 24, 2023
The use of LLMs in healthcare is still quite new, so health systems want to deploy these tools in the least risky way possible. A panel of experts explained how they think health systems can do this during MedCity’s INVEST conference — some of their advice included starting with deployment in nonclinical settings and partnering with incumbent vendors rather than startups.

Fierce Pharma
MAY 24, 2023
Innoviva subsidiary Entasis scores FDA nod for bacterial pneumonia combo drug Xacduro kdunleavy Wed, 05/24/2023 - 09:46

MedCity News
MAY 24, 2023
At a time when the vast majority of people are just beginning to hear and learn about artificial intelligence, it is critical that technology executives make a concerted effort to reinforce its purpose and intent.
Pharma Rep Focus brings together the best content for pharma rep professionals from the widest variety of industry thought leaders.
European Pharmaceutical Review
MAY 24, 2023
The US Food and Drug Administration (FDA) has approved the first-ever redosable gene therapy for the rare skin disease dystrophic epidermolysis bullosa (DEB). VYJUVEK (beremagene geperpavec-svdt) is authorised for DEB patients six months of age or older. The gene therapy is a topical gel that restores functional copies of the COL7A1 gene to provide wound healing and sustained functional COL7 protein expression with redosing.

Fierce Pharma
MAY 24, 2023
Viatris scouts for new board chair as longtime leader Robert Coury transitions to advisory role esagonowsky Wed, 05/24/2023 - 09:26

PharmaTech
MAY 24, 2023
Process development methods & analytical requirements are integral parts of gene therapy manufacturing. This whitepaper explores the analytical and development approaches needed for success.

Pharmaceutical Technology
MAY 24, 2023
Forge Biologics has joined the public-private collaboration, the Bespoke Gene Therapy Consortium (BGTC), to expedite the development and manufacture of new AAV [adeno-associated virus] gene therapies to treat patients with rare diseases. The BGTC is part of the accelerating medicines partnership (AMP) programme and is managed by the Foundation for the National Institutes of Health (FNIH).

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

PharmaTech
MAY 24, 2023
Alvotech and Advanz Pharma have signed a strategic partnership agreement for the supply and commercialization of five biosimilar candidates in Europe.

MedCity News
MAY 24, 2023
Omada’s new program provides coaching, peer support and educational materials to patients struggling with chronic obesity and taking GLP-1s. The company chose not to prescribe the weight loss drugs itself.

European Pharmaceutical Review
MAY 24, 2023
Alvotech and Advanz Pharmaceutical have extended their partnership, regarding the supply and commercialisation of five proposed biosimilars in Europe. “We are very excited to extend our existing partnership with Advanz Pharma into additional therapeutic areas… to provide better patient access to more affordable biologics,” stated Robert Wessman, Chairman and CEO of Alvotech.

MedReps
MAY 24, 2023
Selling yourself is an important part of every job interview. When you go in to speak to a recruiter or human resources worker for a medical sales job interview, you’ll be asked to highlight as many strengths as possible. In order to do this effectively, you need to not only know which strengths are best for the job but also how to illustrate the fact that you have them.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
European Pharmaceutical Review
MAY 24, 2023
Research proposing a digital twin (DT) based approach has outlined the types of information required for components in a laboratory automation plug & play (LAPP) framework. This system provides overall recommendations for integrating robots into life science laboratories. The paper stated that the framework aims to simplify the integration and set-up process for these types of robots by removing the requirement for manual configuration by the user in addition to teaching of the robots.

MedCity News
MAY 24, 2023
Robert Lewis is the founder of Freedom to Roam, a medical device company seeking to help people with mobility challenges.

Copyright Clearance Center
MAY 24, 2023
The post 4 Foundational Copyright Tips for Medical Communications Professionals appeared first on Copyright Clearance Center.

PharmaTimes
MAY 24, 2023
Vital data supports early intervention in order to treat chronic obstructive pulmonary disease

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.
PharmExec
MAY 24, 2023
Treatment to support patients with dystrophic epidermolysis bullosa.

PharmaTimes
MAY 24, 2023
Celadon will sell a minimum of £3m worth of the high-THC product over the next three years

Pharmaceutical Technology
MAY 24, 2023
HBM’s wholly owned subsidiary Nona Biosciences has entered an agreement with OPKO Health’s ModeX Therapeutics for the discovery of antibodies. ModeX Therapeutics will gain access to the fully human Harbour Mice platforms of Nona Bioscience for integration into its MSTAR platform to expedite the monoclonal antibodies’ discovery. This is expected to significantly shorten the preclinical antibody therapeutics development process.

PharmaTech
MAY 24, 2023
As organizations seek to identify areas where technology can make the most impact, a look at some real-world examples of how artificial intelligence (AI) and machine learning (ML) are being used in highly regulated environments can offer valuable insight.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

Clarify Health
MAY 24, 2023
Though difficult, patient journey mapping is an invaluable process for health systems. Having a complete picture of how patients access and move through the health system allows administrators to see how the organization is performing at each touch point. For the patient, this can lead to improved patient experience, better-informed patient outreach efforts, and a higher-quality care experience overall.
PharmaTech
MAY 24, 2023
In this episode of Drug Digest, Pharmaceutical Technology editors discuss what happens in early phase development, the role of CDMOs, and highlight the benefits and challenges of the outsourcing process.
Pharmaceutical Technology
MAY 24, 2023
Y-mAbs Therapeutics has received marketing authorisation for Danyelza (naxitamab-gqgk) 40mg/10mL injection from the Brazilian Health Regulatory Agency, Agência Nacional de Vigilância Sanitária, to treat high-risk neuroblastoma. Danyelza is a recombinant humanised monoclonal antibody that acts on the ganglioside GD2 that is highly expressed in several neuroectoderm-derived tumours and sarcomas.
PharmaTech
MAY 24, 2023
The new CGMP facility is expected to contribute to the development of mRNA therapeutics, with a building designed for the CGMP manufacture of mRNA-based in-vivo gene editing, gene-edited cell therapies, protein replacement therapies, cancer vaccines, and infectious disease vaccines.

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic

Pharmaceutical Technology
MAY 24, 2023
Inovio has received orphan drug designation for INO-3107 from the European Commission (EC) to treat recurrent respiratory papillomatosis (RRP). The investigational DNA medicine candidate INO-3107 has been designed for eliciting a targeted T cell response against HPV[human papillomavirus]-6 and HPV-11, the types of HPV that cause RRP and other related diseases.

Legacy MEDSearch
MAY 24, 2023
iPredict TM AI Eye Screening System provides fully automated age-related macular degeneration (AMD) screening, including retinal imaging and immediate reporting of actionable results. Using the iPredict TM System, primary care and various specialty practices can accurately and efficiently screen people over 50 for AMD and refer them to a specialist (i.e., an ophthalmologist).
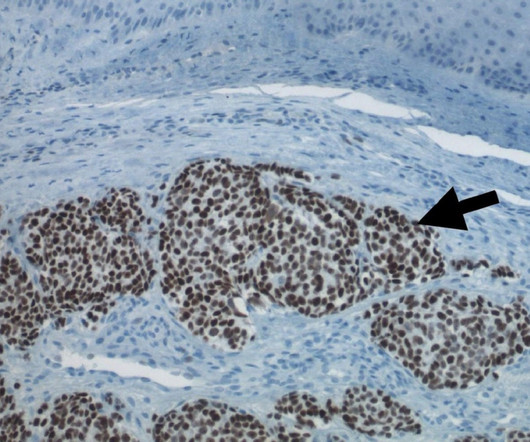
Pharmaceutical Technology
MAY 24, 2023
CohBar has entered into a definitive merger deal with Morphogenesis to advance a late-stage clinical immuno-oncology pipeline of therapies to overcome resistance to cancer immunotherapy. Expected to operate under the name TuHURA Biosciences, the combined company will focus on advancing two technologies of Morphogenesis that seek to overcome the major hurdles which limit existing immunotherapies’ effectiveness in treating cancer.

Integrity Solutions
MAY 24, 2023
What Is Sales Training? Sales Training – at its heart – should be about learning to uncover and identify customer needs, thus creating value and providing service. Selling is about helping customers make buying decisions that are in their best interests. Successful sales people know is about doing something for the customer – not to the customer.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content