Ghana approves University of Oxford's malaria vaccine ahead of key WHO review
Fierce Pharma
APRIL 14, 2023
Ghana approves University of Oxford's malaria vaccine ahead of key WHO review zbecker Fri, 04/14/2023 - 11:13

Fierce Pharma
APRIL 14, 2023
Ghana approves University of Oxford's malaria vaccine ahead of key WHO review zbecker Fri, 04/14/2023 - 11:13
MedCity News
APRIL 14, 2023
A panel discussion at the annual Abarca Forward conference in Puerto Rico last month sought to identify some of the innovative financial approaches that can be applied to managing the high price of novel therapies. Conversations also addressed what payers need for these financing models to be sustainable.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Fierce Pharma
APRIL 14, 2023
FDA advisers back Lundbeck and Otsuka's Rexulti in Alzheimer's disease agitation zbecker Fri, 04/14/2023 - 18:14
MedCity News
APRIL 14, 2023
Ada Health recently announced that Jefferson Health is deploying its technology across its entire enterprise as part of a digital front door initiative. The Berlin-based company’s AI-powered symptom assessment and care navigation tools helps health systems achieve a robust digital front door, which means staff members don’t have to spend as much time triaging patients or helping them navigate the process of finding care.

Fierce Pharma
APRIL 14, 2023
AACR: Patient deaths taint Roche's industry-first early-stage liver cancer readout for Tecentriq and Avastin aliu Fri, 04/14/2023 - 22:19

MedCity News
APRIL 14, 2023
The American Health Care Association and the National Center for Assisted Living reported in June that nearly half of the nation’s 14,000 nursing homes are facing severe labor shortages, and 73 percent of those facilities are concerned about closing.
Pharma Rep Focus brings together the best content for pharma rep professionals from the widest variety of industry thought leaders.

Pharmaceutical Technology
APRIL 14, 2023
Ghana’s Food and Drug Authority (FDA) has approved R21/Matrix-M malaria vaccine in children aged 5 to 36 months, marking the first regulatory clearance for the University of Oxford-developed vaccine in any country in the world. Serum Institute of India (SIIPL), manufacturer and licence holder of the vaccine, has been notified of the registration by Ghana’s FDA.

European Pharmaceutical Review
APRIL 14, 2023
The current progress, challenges and opportunities for achieving stable formulations of nucleic acid (NA) therapeutics with novel drug delivery systems (DDSs) has been discussed in a paper published in Pharmaceutics. Over the past decade, the impact of nucleic acid (NA)-based biopharmaceuticals has been facilitated by breakthroughs associated with high-end manufacturing and pharmaceutical drug delivery.
Pharmaceutical Technology
APRIL 14, 2023
Clinical-stage biopharmaceutical company TORL BioTherapeutics has raised $158m in a Series B financing round for advancing the development of new biologics for cancer treatment. TORL BioTherapeutics is a newly formed, US-based company focused on the development of novel antibody-based therapeutics for cancer patients. Led by Goldman Sachs Asset Management, the financing round has also seen participation from Deep Track Capital, Moore Strategic Ventures, Cowen Healthcare Investments, Bristol Myer

MedCity News
APRIL 14, 2023
Ambient AI promises a second coming for technology at the point of care enabling EHR systems to elegantly work for providers in the background, in natural workflows and “in conversation,” versus requiring the provider and patients to step aside, waste time, and “feed the beasts” of legacy transactional systems.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
Pharmaceutical Technology
APRIL 14, 2023
Ginkgo Bioworks (Ginkgo) and the Wisconsin Alumni Research Foundation (WARF) have collaborated to discover next-generation GD2 CAR T-cell therapies to treat solid tumours. WARF is the patenting and licensing organisation for the University of Wisconsin–Madison in the US. Ginkgo will partner with researchers from Wisconsin-Madison to discover new and improved GD2 CAR designs with improved persistence, fitness and proliferation.

PharmExec
APRIL 14, 2023
By tailoring messages, leveraging digital channels, creating personalized experiences, and fostering relationships, healthcare marketers can connect with HCPs across generations and build lasting partnerships.
Pharmaceutical Technology
APRIL 14, 2023
The US Food and Drug Administration (FDA) has granted rare paediatric drug designations for IPS HEART’s stem cell therapeutics, GIVI-MPC and ISX9-CPC, to treat Duchenne muscular dystrophy (DMD) patients. GIVI-MPC is intended to create new skeletal muscle with 100% full-length dystrophin, while ISX9-CPC is designed to create new functional cardiac muscle for indicated patients.

MedCity News
APRIL 14, 2023
Scene Health’s $17.7 million Series B funding round was led by ABS Capital Partners and includes participation from Claritas Health Ventures, PTX Capital, Kapor Capital and Healthworx, the investment arm of CareFirst BlueCross BlueShield. Since Scene Health was founded in 2014, it has raised more than $25 million total.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
Pharmaceutical Technology
APRIL 14, 2023
Twist Bioscience has introduced Twist T-cell receptor (TCR) and Twist chimeric antigen receptor (CAR) libraries to facilitate the development of cell therapies. Researchers will now be able to speed up the creation of tailor-made libraries that can help in identifying and developing new cell therapies. Both libraries feature up to 10,000 gene fragment combinations, and allow the high-throughput screening and characterisation of new and known sequence variants to develop therapeutic solutions.

MedCity News
APRIL 14, 2023
Biotech IPOs continues to be almost non-existent, but Acelyrin wants to see if the public markets have an appetite for its lead drug candidate. The small protein drug could match up favorably against blockbuster biologics and it has reached pivotal testing in three inflammatory disorders.
Pharmaceutical Technology
APRIL 14, 2023
Biotechnology company Tiziana Life Sciences has revealed plans to assess intranasal foralumab as a potential treatment for long Covid. Long Covid is a health condition characterised by the continuation or development of new symptoms three months after an initial SARS-CoV-2 infection. The work is supported by the role of foralumab in dampening activated microglia cells, said to be an important component of the pathogenesis of this disease.

European Pharmaceutical Review
APRIL 14, 2023
Oligonucleotide therapeutics represent a relatively novel class of drug, with the potential to modulate drug targets that were previously considered intractable, and with the benefit of fast clinical development times. 1 At the WuXi TIDES Forum in March, experts vocalised their opinions on the current trends and challenges they have faced in developing these innovative treatments.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.
PharmaTech
APRIL 14, 2023
Ginkgo Bioworks has acquired StrideBio's AAV capsid discovery and engineering platform and has formed a partnership with WARF for development of next-gen cell therapies.

Copyright Clearance Center
APRIL 14, 2023
The post London Book Fair Expects Strong Showing appeared first on Copyright Clearance Center.

Cesare Ferrari
APRIL 14, 2023
Do you know that customer retention, loyalty, and delight are different? It’s okay if you’re finding out. All three are linked to customer satisfaction and provide substantial benefits for a medical device company. Remember, the goal of any marketing strategy is to attract, satisfy and retain customers in the target segment. This is because the longer customers stay with a company, the more benefits the customer generates.

Pharmaceutical Technology
APRIL 14, 2023
Alvotech has received a complete response letter (CRL) from the US Food and Drug Administration (FDA) regarding its biologics licence application (BLA) for AVT02, a biosimilar to Humira (adalimumab). The CRL stated that the BLA application cannot be approved until deficiencies identified at the company’s manufacturing facility in Reykjavik, Iceland, are satisfactorily resolved.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

Pharma Leaders
APRIL 14, 2023
Caris Life Sciences and Flare Therapeutics have announced a partnership to advance the precision oncology pipeline of the latter into clinical trials. The multi-year strategic partnership will leverage latest molecular profiling approaches guiding patient selection and participation to accelerate precision medicine approaches across five of the therapeutic programmes of Flare.
Pharmaceutical Technology
APRIL 14, 2023
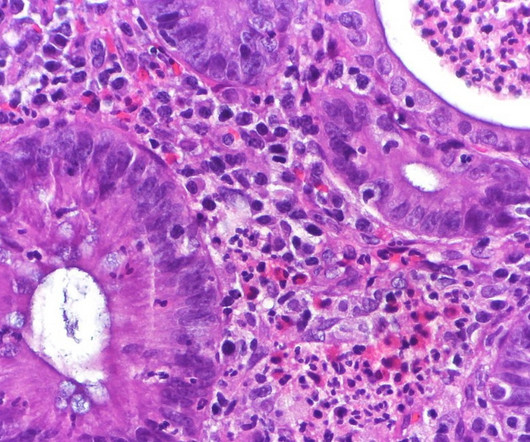
The US Food and Drug Administration (FDA) has rejected Eli Lilly’s biologic licence application (BLA) for the ulcerative colitis (UC) drug mirikizumab over manufacturing concerns. The regulator has issued a complete response letter. No concerns related to the clinical data package, safety or the medicine label. Eli Lilly executive vice-president Patrik Jonsson said: “We remain confident in mirikizumab’s pivotal Phase III clinical data and its potential to help people with ulcerative coliti

Pharma Leaders
APRIL 14, 2023
The FDA has granted Fast Track designation to Regenxbio’s RGX-202 candidate as a potential one-time gene therapy treatment for Duchenne muscular dystrophy. The disorder, which is characterized by progressive muscular weakness, is caused by a genetic mutation that affects the production of dystrophin, resulting in cell damage during muscle contraction.

Pharmaceutical Technology
APRIL 14, 2023
The US Food and Drug Administration (FDA) has granted Fast Track designation to SAB Biotherapeutics’ SAB-176 to treat high-risk Type A and Type B influenza illness patients, including those with antiviral-resistant strains. The new, highly potent neutralising immunoglobulin antibody has been developed for preventing or reducing severe outcomes of Type A and Type B influenza infection in patients at high risk for severe complications, including immunocompromised individuals.

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic

Legacy MEDSearch
APRIL 14, 2023
Neuvotion, Inc. is an early-stage medical device company developing neurostimulation products for the rehabilitation, brain-computer interface, and physical therapy markets, today announced that it has received an additional $1.25M in funding, bringing cumulative funding to over $2.75M. Northwell Holdings, the venture investment arm of Northwell Health, Topspin Fund, and Long Island Angel Network also participated in the round.
Pharmaceutical Technology
APRIL 14, 2023
Madrigal Pharmaceuticals’ resmetirom for nonalcoholic steohepatitis (NASH) could be fairly priced at a higher range than Intercept Pharmaceuticals’ obeticholic acid, based on a revised evidence report published by the Institute for Clinical and Economic Review (ICER). According to ICER’s Health Benefit Price Benchmark (HBPB), resmetirom would be fairly priced between $39,600 and $50,100, while obeticholic acid would be fairly priced between $32,800 and $40,700.

Eversana Intouch
APRIL 14, 2023
When does it make sense to invest in the time, tools, and talent to create or strengthen internal capabilities in your organization? When does it make sense to work with an external partner to gain specialized ability rapidly, and augment your team without major structural changes and long-term commitment? Every situation is unique, and there are compelling reasons to do each, at different moments.

PharmaTech
APRIL 14, 2023
FDA has granted fast track designation to Caribou Biosciences for its allogeneic CAR-T cell therapy for relapsed or refractory multiple myeloma.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content