Eli Lilly damages tripled to $184M in Medicaid rebate fraud case
Fierce Pharma
MAY 10, 2023
Eli Lilly damages tripled to $184M in Medicaid rebate fraud case fkansteiner Wed, 05/10/2023 - 10:25

Fierce Pharma
MAY 10, 2023
Eli Lilly damages tripled to $184M in Medicaid rebate fraud case fkansteiner Wed, 05/10/2023 - 10:25

MedCity News
MAY 10, 2023
Medical Affairs plays a critical role when it comes to driving adherence to evidence-based medicine (EBM) – the principle that clinical decisions should be informed by the best available scientific evidence, along with clinical experience and patient preference.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Fierce Pharma
MAY 10, 2023
Novartis targets underserved fields—and CAR-T—for next phase of immunology growth: exec aliu Wed, 05/10/2023 - 12:08
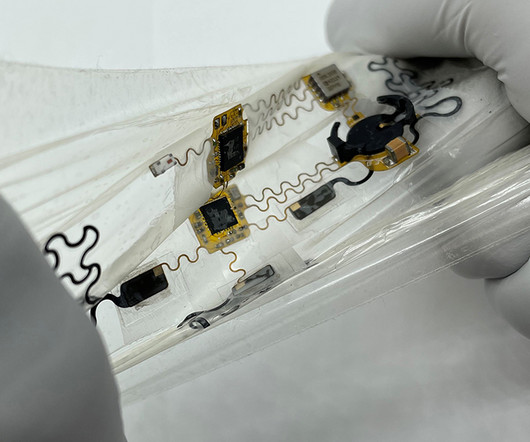
Medgadget
MAY 10, 2023
Researchers at the University of Texas at Austin have developed a new chest wearable that can obtain both electrocardiogram and seismocardiogram data from the underlying heart. While basic ECG can be monitored via smart watches, no other wearable combines it with seismocardiography, which would conventionally be obtained by listening to the heart using a stethoscope.

Fierce Pharma
MAY 10, 2023
Sandoz taps Evotec in long-term biosimilar development and manufacturing deal fkansteiner Wed, 05/10/2023 - 08:39

MedCity News
MAY 10, 2023
Regulators have become increasingly concerned about the potential for medical devices to become a vector for spreading malware attacks across hospital networks, resulting in untold patient harm and billions of dollars globally.
Pharma Rep Focus brings together the best content for pharma rep professionals from the widest variety of industry thought leaders.

Pharmaceutical Technology
MAY 10, 2023
Gilead Sciences has emerged victorious in a legal battle with the US government over patents surrounding the HIV pre-exposure prophylaxis (PrEP) drugs Descovy and Truvada following a federal jury’s verdict on May 9. A jury in the Delaware District Court gave a favourable verdict to the Foster City, California-headquartered company, concluding that Gilead did not infringe any of the US government’s patents.

Fierce Pharma
MAY 10, 2023
Bernie Sanders-led Senate committee takes pharma and PBMs to task—even after insulin price cuts zbecker Wed, 05/10/2023 - 17:11
Pharmaceutical Technology
MAY 10, 2023
Synlogic has received orphan drug designation (ODD) from the US Food and Drug Administration (FDA) for SYNB1934 to treat phenylketonuria (PKU), a rare inherited metabolic disease. The orally administered, non-systemically absorbed drug candidate SYNB1934 has been designed for reducing blood phenylalanine (Phe) levels in PKU patients. SYNB1934 consumes Phe in the gastrointestinal (GI) tract by leveraging genetic engineering of the drug or drug-carrying capsule, probiotic escherichia coli (E coli)

Fierce Pharma
MAY 10, 2023
Teva CEO Richard Francis, after 4 months at the helm, preps grand strategic reveal zbecker Wed, 05/10/2023 - 11:26

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

MedCity News
MAY 10, 2023
Traditional fertility benefit practices — like dollar cap benefits and step therapy — are creating inequities in treatment, said Ann Gaines, senior vice president of business development at Progyny. Gaines made these comments Wednesday during the Midwest Business Group on Health conference in Chicago.

Fierce Pharma
MAY 10, 2023
Defense Department backs new study of Idorsia's Quviviq as a treatment for PTSD kdunleavy Wed, 05/10/2023 - 07:07
Spotio
MAY 10, 2023
Business-to-business (B2B) prospecting is the pillar of lead generation. When prospecting for new business, the goal is to identify as many quality prospects as possible. However, B2B sales prospecting strategy is not as easy as it sounds. According to Technology Advice , 70% of marketers at B2B companies reported that “improving lead quality” was the primary concern.

Fierce Pharma
MAY 10, 2023
Sobi snatches up CTI and blood cancer drug Vonjo in $1.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

PharmExec
MAY 10, 2023
According to the latest State of Revenue survey of c-suite executives, pharma manufacturers are prioritizing digital transformation while struggling with inflation and supply chain disruptions.

MedCity News
MAY 10, 2023
A drug developed by Protalix BioTherapeutics and Chiesi Group is now FDA approved for treating Fabry disease, a rare inherited metabolic disorder. The drug, Elfabrio, is an enzyme replacement therapy.

Medgadget
MAY 10, 2023
Researchers at MIT have developed an ‘electroceutical’ capsule that is designed to be swallowed and which will deliver a small electrical current to the stomach wall. The device contains an external electrode that wraps around its exterior and small grooves that draw liquid away from the electrode and help it to contact the stomach wall.

European Pharmaceutical Review
MAY 10, 2023
Sandoz has announced it is investing €25 million in its manufacturing site in Holzkirchen, Germany, to expand its biosimilar development capabilities. The company intends to grow its Biopharma Technical Development (BioTD) capabilities and transform the lab building into a state-of-the-art biotech lab by the last quarter of 2023. The financing is intended to bring together these highly advanced laboratories and analytical expertise at one site.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.

PharmExec
MAY 10, 2023
BCG’s 2022 benchmark study uncovers new competencies needed for pharma access leaders in enterprise-wide business strategy, where thoughtfully designed approaches and goals focused on shared priority and responsibility are increasingly important.

PharmaVoice
MAY 10, 2023
With decades of experience in public health, Boone is leveraging RWE to move the needle within clinical development.
MedCity News
MAY 10, 2023
“Precision medicine” is a term we have heard a lot over the last two decades, […]

PharmaTimes
MAY 10, 2023
Transfer marks start of vital development stage as company expands its range of synthesized DNA

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

MedCity News
MAY 10, 2023
Learn how one healthcare benefit manager in the insurance space values virtual primary care.

PharmaTimes
MAY 10, 2023
Patients living with the debilitating impacts of migraine attacks will be able to access Vydura
Pharmaceutical Technology
MAY 10, 2023
Swedish Orphan Biovitrum (Sobi) has entered into a definitive agreement to buy biopharmaceutical company CTI BioPharma in an all-cash deal valued at $1.7bn. Sobi will start a tender offer through its wholly owned indirect subsidiary to buy all outstanding CTI shares for $9.10 for each share of common stock in cash. The acquisition will strengthen Sobi’s haematology medicines portfolio with the addition of CTI’s lead product Vonjo (pacritinib), a novel oral kinase inhibitor of JAK2, IRAK1 a

Scott’s Directories
MAY 10, 2023
If you’re trying to sell a product or service to physicians in Alberta, you know that it can be a challenging task. With so many healthcare providers in the province, it can be difficult to know where to start. However, one of the most effective ways to make sales in this industry is by using a list of targeted physicians. In this blog post, we’ll share some tips and strategies for making sales with a list of physicians in Alberta.

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic

Legacy MEDSearch
MAY 10, 2023
Sibel Health, an award-winning digital health company spun out of the Querrey Simpson Institute for Bioelectronics at Northwestern University, announces a new 510(k) clearance from the U.S. Food and Drug Administration (FDA) for continuous neonatal and infant monitoring for babies born of any gestational age to infants of 2 years at the International Maternal Newborn Health Conference in Cape Town, South Africa.
Pharmaceutical Technology
MAY 10, 2023
Amgen will use TScan’s target discovery platform to identify potential targets for Crohn’s disease. As part of the multi-year collaboration , TScan will receive $30m upfront and a possible $500m in clinical, regulatory, and commercial milestones, in addition to royalty payments. TScan’s TargetScan identifies natural targets of T cell receptors. Amgen will use the technology to create novel therapeutics for Crohn’s disease.

LEVO Health
MAY 10, 2023
With strict regulations and a rapidly evolving landscape, healthcare marketing requires a diverse and skilled team. However, with the right approach, you can create a strong marketing strategy that not only complies with regulations but also enhances patient engagement and drives growth for your practice. Understanding the Regulatory Landscape To create a successful healthcare marketing strategy, it’s essential to familiarize yourself with the relevant regulations, such as: Health Insuranc
Pharmaceutical Technology
MAY 10, 2023
Roche has acquired the worldwide rights to develop and commercialise Chinese biotechnology company Zion Pharma’s lead programme, ZN-A-1041. Roche will handle the global development, manufacturing and commercialisation activities of ZN-A-1041, after an initial transition period. Zion Pharma will receive an upfront payment of $70m, along with near-term milestone payments.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content