Sickle cell disease gene therapies from Vertex, bluebird can be cost-effective at $1.9M: ICER
Fierce Pharma
APRIL 13, 2023
Sickle cell disease gene therapies from Vertex, bluebird can be cost-effective at $1.

Fierce Pharma
APRIL 13, 2023
Sickle cell disease gene therapies from Vertex, bluebird can be cost-effective at $1.

MedCity News
APRIL 13, 2023
Virtual reality and podcasts are two innovative tools that can help captivate your audience and differentiate your healthcare brand. By utilizing these cutting-edge technologies, you can stay ahead of the competition and create more engaging marketing campaigns.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Fierce Pharma
APRIL 13, 2023
With Wegovy supply unfettered, Novo Nordisk ratchets up its 2023 growth forecast fkansteiner Thu, 04/13/2023 - 17:23

European Pharmaceutical Review
APRIL 13, 2023
Psychedelics are a class of psychoactive substances that produce temporary changes in perception, mood and cognitive processes. 1 Some psychedelics are found in nature, including psilocybin, a naturally occurring molecule found in almost 150 species of mushrooms which turns into psilocin once ingested, causing psychedelic effects. Other psychedelics are man-made, such as lysergic diethylamide (LSD), which was first synthesised in 1938 by Albert Hofmann at a laboratory in Switzerland. 2 LSD is wi

Fierce Pharma
APRIL 13, 2023
Otsuka, Lundbeck head into key FDA panel meeting with agency support for their Rexulti application esagonowsky Thu, 04/13/2023 - 08:47

MedCity News
APRIL 13, 2023
Hospital M&A activity remained strong during the first quarter of 2023, according to a new report from Kaufman Hall. Hospitals and health systems announced 15 M&A transactions in the first quarter of 2023, which almost matches the 17 transactions announced in the fourth quarter of 2022.
Pharma Rep Focus brings together the best content for pharma rep professionals from the widest variety of industry thought leaders.
Pharmaceutical Technology
APRIL 13, 2023
Dyadic International has announced expansion of a licence agreement for its C1-cell protein expression platform with South African consortium Rubic One Health. The expanded licence will include the development of vaccines and therapeutic proteins beyond Covid-19 for human and animal health markets in Africa. During the Covid-19 pandemic, vaccination rates of many countries in Africa were significantly trailing the rest of the world.
Fierce Pharma
APRIL 13, 2023
Fierce Pharma Asia—Takeda's gene therapy pivot and pay-for-delay lawsuit; Astellas' write-offs aliu Thu, 04/13/2023 - 16:07

MedCity News
APRIL 13, 2023
Mifepristone and misoprostol, two pills that are taken together to terminate a pregnancy, have been approved by the FDA since 2000. But access to these drugs have been in serious jeopardy this year, and a federal judge in Texas recently ruled to suspend the FDA’s decadeslong approval of mifepristone. The final outcome for abortion pill access in the U.S. is still unclear among confliction federal rulings — the issue will likely end up in the Supreme Court.

Fierce Pharma
APRIL 13, 2023
'The Top Line': The biotechs that scored top VC dollars in 2022, plus this week's headlines tcarey Thu, 04/13/2023 - 14:27

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
MedCity News
APRIL 13, 2023
Rejection of Eli Lilly’s mirikizumab means that two of the four drugs the pharma giant identified as revenue growth drivers for 2023 have failed to pass the regulatory bar. One of those drugs has been approved while the fourth drug still awaits an FDA decision.

Fierce Pharma
APRIL 13, 2023
Clinical Trial Training Delivery for Remote Audiences mteefey Thu, 04/13/2023 - 11:35
ALULA
APRIL 13, 2023
Recently I attended the Women in Leadership panel at the American Manufacturing Summit. The panel included senior leaders from Caterpillar, Cummins, The Boeing Company, Kimberly-Clark Corporation, Philips, and Johnson & Johnson. The conversation focused on sharing personal experiences - both accomplishments and challenges - about being a woman and an executive in male-dominated industries.

Fierce Pharma
APRIL 13, 2023
GSK throws counterpunch as 'skinny' label feud with Teva circles Supreme Court fkansteiner Thu, 04/13/2023 - 10:05

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

Pharmaceutical Technology
APRIL 13, 2023
Takeda has announced that it will be pivoting away from its discovery and preclinical programmes in adeno-associated virus (AAV) gene therapies. The company is aiming to refocus its efforts on its core therapeutic areas and current late-stage programmes such as the oral TYK2 inhibitor TAK-279, which is under development for several autoimmune disorders.

MedCity News
APRIL 13, 2023
Papa’s Social Care Navigation offering provides users with a team of social care navigators, including social workers, registered nurses, case managers and dieticians. This team can help with social issues the patient may be experiencing, including housing, paying for groceries and overdue health screenings. After only offering the program to a select group of health plan clients last year, Social Care Navigation is now available to all of Papa’s payer customers.

European Pharmaceutical Review
APRIL 13, 2023
INTERPHEX 2023 is right around the corner, taking place April 25-27 at the Javits Center in NYC. INTERPHEX is set to bring leading brands and professionals together once again with new features, a reimagined Show Floor and returning Show Floor favourites. From development to distribution, INTERPHEX is the leading global event that takes you through all stages of the pharmaceutical product development lifecycle.

MedCity News
APRIL 13, 2023
The Blue Cross Blue Shield Association announced it is partnering with Cyversity, a nonprofit working to create a diverse and inclusive workforce in the cybersecurity field. The two organizations are forming a training and mentorship program available to Blue Cross and Blue Shield companies and Cyversity members.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.

European Pharmaceutical Review
APRIL 13, 2023
A new automated buffer management system (BMS) that includes buffer volume monitoring, automatic ordering, preparation and delivery of buffers to the buffer-consuming purification systems has been developed by Swedish researchers. The study from Lund University reconfigured a chromatography system to perform buffer formulation. Buffer management in biopharmaceutical purification is usually performed manually in small scale operations.

MedCity News
APRIL 13, 2023
As margins at health systems continue to contract, and insurance company profits continue to surge, contract negotiations are becoming increasingly contentious. With billions of dollars potentially at stake, you need to be prepared and aligned well in advance.

Copyright Clearance Center
APRIL 13, 2023
The post Discussing Data Challenges and Solutions at the 2023 London Book Fair appeared first on Copyright Clearance Center.
MedCity News
APRIL 13, 2023
The new year always brings hope, but this January that hope was palpable for the […]

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.
MedCity News
APRIL 13, 2023
Three months after Alentis Therapeutics’ lead program posted positive Phase 1 data, the biotech has raised cash to advance that program to mid-stage testing in fibrosis. The Series C financing will also support a cancer drug candidate ready to start human testing.

PharmaTimes
APRIL 13, 2023
Notable first clearance for R21/Matrix-M malaria vaccine for use in a specific country
Pharmaceutical Technology
APRIL 13, 2023
Fusion Pharmaceuticals (Fusion) has received clearance from the US Food and Drug Administration (FDA) for its investigational new drug (IND) applications for [225Ac]-FPI-2068 (FPI-2068) and corresponding imaging analogue [111In]-FPI-2107 (FPI-2107). FPI-2068 is a bispecific targeted alpha therapy (TAT) being jointly developed by Fusion and AstraZeneca under a multi-asset collaboration agreement.

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic

PharmaTimes
APRIL 13, 2023
Decision will create opportunity for therapy to be used earlier in the treatment pathway
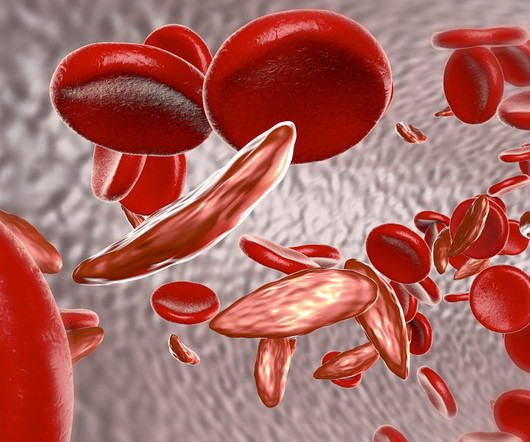
Pharmaceutical Technology
APRIL 13, 2023
Two gene therapies up for approval this year for sickle cell disease could be cost effective in some cases at a $2 million price point, based on a draft evidence report published by the Institute for Clinical and Economic Review (ICER). Released on April 12, the report focuses on bluebird bio’s lovotibeglogene autotemcel and Vertex Pharmaceuticals and CRISPR Therapeutics’ exagamglogene autotemcel or exa-cel and their potential use in treating sickle cell disease.
PharmaVoice
APRIL 13, 2023
The COVID-19 pandemic highlighted an ongoing problem of vaccine hesitancy undermining public health efforts. One way pharmas can help is to change the narrative.

PharmaTech
APRIL 13, 2023
The combination of modular facilities and closed processing offers significant advantages in the production of biopharmaceuticals and is becoming a compelling option for manufacturing.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content