After Johnson & Johnson loses again in bankruptcy case, it's game on for talc lawsuits
Fierce Pharma
APRIL 3, 2023
After Johnson & Johnson loses again in bankruptcy case, it's game on for talc lawsuits kdunleavy Mon, 04/03/2023 - 06:44

Fierce Pharma
APRIL 3, 2023
After Johnson & Johnson loses again in bankruptcy case, it's game on for talc lawsuits kdunleavy Mon, 04/03/2023 - 06:44

MedCity News
APRIL 3, 2023
Hospitals are being more careful than ever when scrutinizing ROI for new technology. Ashis Barad — Allegheny Health Network’s chief information and digital officer — gave advice for health systems follow during the adoption process for new technology, such as ensuring clinicians are involved early on and viewing Big Tech as partners instead of threats.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Fierce Pharma
APRIL 3, 2023
After rumored merger fell through, Merck and Seagen's Padcev-Keytruda combo wins bladder cancer nod fkansteiner Mon, 04/03/2023 - 18:10
Pharmaceutical Technology
APRIL 3, 2023
The World Health Organisation (WHO) has revised its recommendations regarding the use of Covid-19 vaccines following a meeting of the agency’s Strategic Advisory Group of Experts on Immunisation (SAGE). The latest guidance applies to the current phase of the pandemic and reflects the impact of the Omicron variant, which has led to high levels of immunity in all age groups through both vaccination efforts and infections across the globe.
Fierce Pharma
APRIL 3, 2023
Amid dispute with FDA, Akebia proposes new dosing for oral anemia drug in kidney disease aliu Mon, 04/03/2023 - 11:12

MedCity News
APRIL 3, 2023
Hear from healthcare executives such as Dave Jacobs, co-founder of Homethrive, EY Global Health Leader Aloha McBride, and Worksite Labs CEO Gary Frazier on how they are meeting the challenges of healthcare innovation.
Pharma Rep Focus brings together the best content for pharma rep professionals from the widest variety of industry thought leaders.

MedCity News
APRIL 3, 2023
The ViVE conference in Nashville, powered by HLTH and CHIME, offered an overview of the latest developments in health tech spanning interoperability, cybersecurity, price transparency, behavioral health and health equity.

Fierce Pharma
APRIL 3, 2023
Viatris maintains legal winning streak with yet another EpiPen antitrust victory fkansteiner Mon, 04/03/2023 - 10:09
Pharmaceutical Technology
APRIL 3, 2023
The European Commission (EC) has granted marketing authorisation for Sandoz’s biosimilar Hyrimoz (adalimumab) citrate-free high-concentration formulation (HCF). Hyrimoz has been approved for use in all the indications covered by the reference medicine Humira, including plaque psoriasis, rheumatic diseases, ulcerative colitis, Crohn’s disease, uveitis and hidradenitis suppurativa.

Fierce Pharma
APRIL 3, 2023
CSL's new CEO Paul McKenzie charts course for growth amid expansions in gene therapy, kidney diseases kdunleavy Mon, 04/03/2023 - 07:44

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

MedCity News
APRIL 3, 2023
The Federal Trade Commission has ordered Illumina to divest Grail, the liquid biopsy company it acquired for more than $7 billion. The agency said Illumina’s proposed remedies are insufficient and the tie-up is likely to reduce competition in the R&D and commercialization of new cancer tests.

Pharmaceutical Technology
APRIL 3, 2023
Sartorius , through its French listed sub-group Sartorius Stedim Biotech , has signed an agreement to acquire Polyplus for €2.4bn ($2.6bn). The deal will see Polyplus join the German life science group’s portfolio allowing the latter to leverage expertise in transfection reagents and plasmid DNA for gene therapy. Polyplus, based in Strasbourg, France, produces key components in the production of viral vectors used in cell and gene therapies.

MedCity News
APRIL 3, 2023
Over 800 women died from pregnancy-related complications in 2020 in the United States, and well […]
Pharmaceutical Technology
APRIL 3, 2023
BioNTech has signed exclusive licence and collaboration agreements with Duality Biologics (DualityBio) for the development of two antibody-drug conjugate (ADC) assets for solid tumours. The agreements also include the manufacturing and commercialisation of the two assets, including DB-1303 and DB-1311, across the globe. As part of the new deals, BioNTech will have commercial rights to the ADCs worldwide, excluding mainland China, the Hong Kong special administrative region and the Macau special

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud

MedCity News
APRIL 3, 2023
Florida healthcare providers are prone to experience unpredictable changes in the revenue cycle when Medicaid plans begin the redetermination, renewal, and disenrollment process.
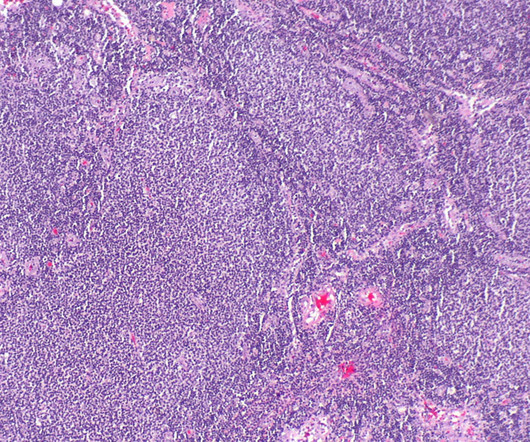
Pharmaceutical Technology
APRIL 3, 2023
Eisai and the National Cancer Center have signed an agreement to partner on investigator-initiated clinical research for the anti-cancer agent, tazemetostat (Tazverik Tablets 200 mg), based on the Patient-Proposed Healthcare Services system. Under this system, patients can request the government for access to medical treatment using unapproved drugs not covered by insurance.

Clear Pivot
APRIL 3, 2023
Transcript: So I'll click on Sign In, and we'll go over to Apple Business Connect. So it's just businessconnect.apple.com. And then I'll go sign in here. So this is our ClearPivot business profile. Our profile was filled out previously, but they've added some new features with this expansion of the platform. I'm on the ClearPivot business info itself here, and so this is basically just like the legal stuff.
Pharmaceutical Technology
APRIL 3, 2023
Ablaze Pharmaceuticals is set to develop a new GPC3-targeted peptide drug candidate for the treatment of liver cancer in China. The company is licensing the first-in-class drug candidate under an existing deal with RayzeBio. The agreement allows Ablaze to clinically develop and commercialise the drug in Greater China. It is based on RayzeBio’s specialisation in targeted radiotherapy (TRT) discovery and Ablaze’s know-how in therapeutic product clinical development, as well as on the radiopharmace

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.

MedCity News
APRIL 3, 2023
As health systems roll out new patient-facing technologies, they need to ensure that their patients are comfortable using these tools. To avoid losing patient trust, hospitals should introduce new technology with transparency and patient education at the forefront of their minds, said Aaron Miri, Baptist Health’s chief digital and information officer.
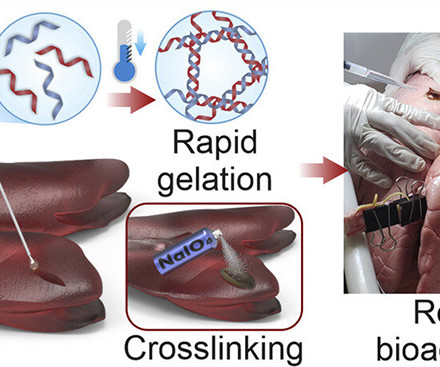
Medgadget
APRIL 3, 2023
Researchers at the Terasaki Institute for Biomedical Innovation in Los Angeles have developed a gelatin-based surgical sealant. The sealant is thermoresponsive, meaning that it will rapidly form a semi-solid bolus when it reaches body temperature. It is also bioadhesive, adhering to slippery, wet surfaces in the body with relative ease. The researchers achieved this by incorporating caffeic acid, a substance that is naturally found in coffee and olive oil, into the gelatin gel, which helped to i

MedCity News
APRIL 3, 2023
BioNTech’s alliance with DualityBio gives it two antibody drug conjugate (ADC) candidates, the most advanced of which is in mid-stage clinical development for solid tumors. It addresses the same target as a potential blockbuster AstraZeneca drug.

European Pharmaceutical Review
APRIL 3, 2023
Nine new medicines were recommended for approval in the European Medicines Agency (EMA)’s Committee for Medicinal Products for Human Use (CHMP) March meeting, Medicines recommended for approval at the CHMP meeting The CHMP recommended authorising the COVID-19 vaccine Bimervax (previously COVID-19 Vaccine HIPRA) as a booster in individuals over 16 years old, who have previously been vaccinated with a mRNA COVID-19 vaccine.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

PharmaTimes
APRIL 3, 2023
Study demonstrated a reduction in viral replication in the upper respiratory tract as well as influenza infection
European Pharmaceutical Review
APRIL 3, 2023
Lorlatinib has shown to be safe and effective in treating high-risk neuroblastoma in a Phase I trial. These results have led to a major amendment in a European Phase III paediatric clinical trial. In the Phase I New Approaches to Neuroblastoma Therapy (NANT) trial, researchers found that lorlatinib given alone or in combination with chemotherapy was safe and tolerable in patients with relapsed/refractory anaplastic lymphoma kinase (ALK)-driven neuroblastoma.

PharmaVoice
APRIL 3, 2023
New reports highlight the need for more targeted biotech education curriculums to shore up a sustainable workforce.

MedReps
APRIL 3, 2023
When it comes to showing your leadership experience on your medical sales resume, it’s important to use the right words. Since many recruiters and human resources workers use either a computerized system to scan and sort resumes or quickly skim the documents themselves, you need to make sure you have the right keywords in place to show you possess the medical sales leadership experience they’re seeking.

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic

Copyright Clearance Center
APRIL 3, 2023
The post ChatGPT & Information Integrity appeared first on Copyright Clearance Center.

PharmaTimes
APRIL 3, 2023
Five vital new appointments made to regulator’s insight, perspective and advice group
MedCity News
APRIL 3, 2023
Data can help healthcare facilities improve patient safety, but first, healthcare leaders need to bring data from multiple sources together using a common taxonomy.
PharmExec
APRIL 3, 2023
EVERSANA honored for innovative use of artificial intelligence in video production — enhancing educational content development for the life science industry.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content