Krystal's Vyjuvek becomes first topical gene therapy with FDA nod to treat rare skin disease
Fierce Pharma
MAY 19, 2023
Krystal's Vyjuvek becomes first topical gene therapy with FDA nod to treat rare skin disease zbecker Fri, 05/19/2023 - 16:25

Fierce Pharma
MAY 19, 2023
Krystal's Vyjuvek becomes first topical gene therapy with FDA nod to treat rare skin disease zbecker Fri, 05/19/2023 - 16:25
Pharmaceutical Technology
MAY 19, 2023
The US Food and Drug Administration (FDA) has granted approval to Bausch + Lomb and Novaliq’s Miebo (perfluorohexyloctane ophthalmic solution) to treat the signs and symptoms of dry eye disease (DED). Formerly known as NOV03, Miebo is a first-in-class eye drop designed for preventing the evaporation of excessive tears and restoring tear balance in evaporative DED patients.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Fierce Pharma
MAY 19, 2023
Intercept's NASH dreams may be dashed after FDA panel votes against Ocaliva's approval bid fkansteiner Fri, 05/19/2023 - 17:30

MedCity News
MAY 19, 2023
Included Health and DispatchHealth are partnering to combine Included’s virtual care services with DispatchHealth’s home care services. If an Included physician notices a patient requires in-person support, the physician will send DispatchHealth to the patient’s home.

Fierce Pharma
MAY 19, 2023
Bausch + Lomb bags FDA approval for dry eye disease treatment Miebo zbecker Fri, 05/19/2023 - 09:39

MedCity News
MAY 19, 2023
Bausch + Lomb drug Miebo is now FDA approved as a new treatment for dry eye disease. Unlike many products that rewet the eye, Miebo is designed to address one of the factors that leads to dry eyes.
Pharma Rep Focus brings together the best content for pharma rep professionals from the widest variety of industry thought leaders.

Pharmaceutical Technology
MAY 19, 2023
The US Food and Drug Administration (FDA) has approved AbbVie’s Rinvoq (upadacitinib) for patients with Crohn’s disease who do not respond to TNF blockers, a common immune suppressant treatment for the condition. Whilst there is a range of FDA-approved biologics for Crohn’s disease, Rinvoq is the first approved oral product for the moderate to severe type of the disease.

Fierce Pharma
MAY 19, 2023
As Catalent delays earnings again, CEO notes financial performance has fallen 'significantly short' fkansteiner Fri, 05/19/2023 - 11:14

MedCity News
MAY 19, 2023
A group of labor unions have filed an antitrust complaint against UPMC. They alleged that the health system has prevented its workers from being able to advocate for themselves and their patients through “a draconian system of mobility restrictions and widespread labor law violations that lock in sub-competitive pay and working conditions.

Fierce Pharma
MAY 19, 2023
At request of FTC, Amgen agrees to delay closure of $27.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
European Pharmaceutical Review
MAY 19, 2023
A Phase III trial has found that the novel combination antibiotic sulbactam-durlobactam prevents at least as many fatalities of hospital-acquired pneumonia as colistin, the best currently approved treatment. This finding alongside the other data from the trial led the US Food and Drug Administration (FDA)’s Antimicrobial Drugs Advisory Committee to recommend the FDA approve the combination antibiotic for often-fatal pneumonia strain carbapenem-resistant Acinetobacter baumannii–calcoaceticus co
Pharmaceutical Technology
MAY 19, 2023
Myeloid Therapeutics has raised $73m to support the continued clinical development of its lead cell therapy programme, MT-101, in Phase I/II trials for T cell lymphoma. Led by Hatteras Investment Partners, the financing round has seen participation from existing investors, including 8VC, Alexandria Venture Investments and Newpath Partners, along with new investors Moore Strategic Ventures and ARCH Venture Partners.
European Pharmaceutical Review
MAY 19, 2023
Final draft guidance has been published for the first National Institute for Health and Care Excellence (NICE)-recommended treatment for symptomatic chronic heart failure with preserved or mildly reduced ejection fraction. The regulatory body’s decision means up to 150,000 patients would be eligible for AstraZeneca-made dapagliflozin (Forxiga).
Pharmaceutical Technology
MAY 19, 2023
Multiple sclerosis (MS) is a primary autoimmune disease in which inflammation is a core contributor to the degeneration of the central nervous system (CNS), leading to neurological disability and affecting sensory, visual, motor, and autonomic systems. While MS is not a terminal diagnosis, the effect of the disease on the CNS can significantly impact patients’ independence and disturb their daily lives.

Advertisement
Planning on running clinical trials in Japan? How can you reliably supply these studies? Discover Catalent’s clinical supply packaging facility in Shiga, Japan. Strategically located between Tokyo and Osaka, and one of largest in Japan, this 6,000 square meter facility offers comprehensive services including primary and secondary clinical packaging and labelling, comparator sourcing, cold chain storage, local and global distribution, local language support and white glove service to support stud
Medgadget
MAY 19, 2023
Scientists at the Japan Advanced Institute of Science and Technology in Ishikawa, Japan have developed an anti-cancer treatment that consists of bacteria that are naturally found inside some tumors. Isolating and then injecting these bacteria into existing tumors appears to provoke a strong immune response that can lead to tumor destruction, without the need for advanced techniques such as bacterial genetic engineering or complex drug delivery.

Copyright Clearance Center
MAY 19, 2023
The post PRH, PEN America Fight “Unconstitutional” School Book Bans appeared first on Copyright Clearance Center.
Medgadget
MAY 19, 2023
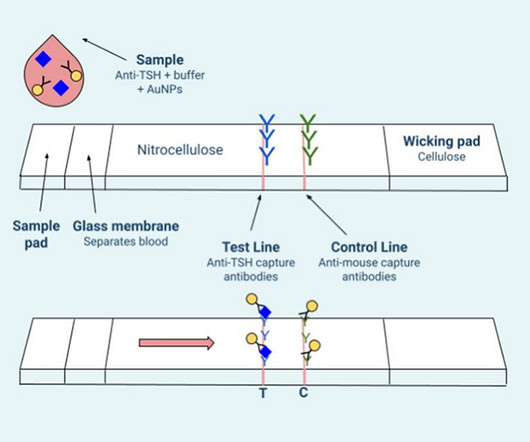
Student researchers at Rice University have developed a paper strip test for hypothyroidism, similar to those that are commonly used for COVID-19 testing. Babies with congenital hypothyroidism require prompt diagnosis and treatment, but in many parts of the world a lack of healthcare resources can mean that the condition can go undiagnosed for long periods, affecting a child’s development.

PharmaTimes
MAY 19, 2023
Screening patients to assess their clopidogrel resistance status will allow for alternative treatments

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.

MedCity News
MAY 19, 2023
Companies presenting their technology at the conference seek to support a diverse patient population spanning conditions such as inflammatory bowel disease, food allergies, and cancer. Cell, gene therapy and immunotherapies also form some of their treatment approaches in development.

PharmaTimes
MAY 19, 2023
Treatment concerns up to 150,000 patients in England with chronic heart failure
PharmaVoice
MAY 19, 2023
Pharma leaders share what they do to keep their teams from fizzling out.

Pharmaceutical Commerce
MAY 19, 2023
Document reveals that 20 of 28 of participating Big Pharma manufacturers saw their DIO increase by an average of 5%, suggesting that large amounts of inventory do not necessarily offer protection from running low on medication.

Advertisement
When selecting a clinical supply provider, consideration often focuses upon the manufacturing, packaging, storage and distribution capabilities available that will, at face-value, be sufficient to meet the needs of the sponsor and their trial. However, there are human-based and knowledge-driven factors that are often overlooked that go beyond these basic physical capabilities and are integral to the development and delivery of high performing clinical supply chains.

Pharmaceutical Technology
MAY 19, 2023
The US Food and Drug Administration (FDA) has accepted Satsuma Pharmaceuticals’ 505(b)(2) new drug application (NDA) for STS101 for acute treatment of migraine, for review. The new investigational therapeutic product candidate STS101 is a nasal powder formulation of dihydroergotamine mesylate (DHE), an anti-migraine drug, which is given through the company’s nasal delivery device.

Scott’s Directories
MAY 19, 2023
In the digital age, lead generation has become a crucial part of every business strategy, and the healthcare industry is no exception. Healthcare professionals face unique challenges when it comes to attracting the right leads, which requires specialized tools and strategies. In this blog post, we will explore how healthcare lead generation companies like MD Select can help healthcare businesses generate quality leads to grow their practices.

Pharmaceutical Technology
MAY 19, 2023
The US Supreme Court has unanimously voted in favour of Sanofi and Regeneron in a years-long legal feud with Amgen over the potential patent infringement surrounding the companies’ anti-cholesterol drugs. This marks a milestone in the dispute nearly a decade after the first lawsuit filed by Amgen against the two companies. After previously receiving the arguments from both companies on March 27, the Supreme Court delivered its verdict on May 18.

PharmaTech
MAY 19, 2023
The UK’s National Institute for Health and Care Excellence has recommended dapagliflozin (Forxiga) from AstraZeneca as a treatment option for adults with symptomatic chronic heart failure with preserved or mildly reduced ejection fraction.

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic

Pharmaceutical Technology
MAY 19, 2023
Alimera Sciences has acquired the US commercialisation rights for Yutiq (fluocinolone acetonide intravitreal implant) 0.18mg from EyePoint Pharmaceuticals. Yutiq is indicated to treat chronic, non-infectious uveitis that affects the posterior segment of the eye. It received approval from the US Food and Drug Administration in October 2018. Alimera Sciences has exclusive global rights to Yutiq excluding Southeast Asia, Hong Kong, Macau, China and South Korea, where the therapy was previously lice

PharmaTech
MAY 19, 2023
Shabbir Mostafa, head of Business Development — Advanced Delivery Systems, Recipharm, sat down with Grant Playter, associate editor, during DCAT week to discuss the current state of inhalations forms and drug delivery.

Pharmaceutical Technology
MAY 19, 2023
SiteOne Therapeutics has received a $15m grant from the National Institute on Drug Abuse (NIDA) to develop the investigational selective NaV1.8 inhibitor, STC-004, as a non-opioid therapeutic for acute and chronic pain conditions. The funding was awarded through the National Institutes of Health’s (NIH) Helping to End Addiction Long-term Initiative (NIH HEAL), a trans-agency effort for the development of scientific solutions to the US national opioid public health crisis.
Pharma Leaders
MAY 19, 2023
Cara Therapeutics and Vifor Fresenius Medical Care Renal Pharma (VFMCRP) have announced that the US’s National Institute for Health and Care Excellence (NICE) has recommended Kapruvia (difelikefalin) to treat chronic kidney disease (CKD)-associated pruritus. Kapruvia has been recommenced to treat moderate-to-severe CKD-associated pruritus in adult patients who are undergoing haemodialysis.

Advertisement
As the development pipeline for new drugs continues to grow, biopharmaceutical companies are re-evaluating how to best manage and balance resources across an increasing number of development projects and complex clinical trials. There are two approaches that can be used to speed a drug from development to clinic faster: timeline compression and parallel processing, but only one that considers the benefits of integrating clinical supply into the overall drug development process.
Let's personalize your content