US FDA approves Bausch + Lomb and Novaliq’s DED treatment Miebo
Pharmaceutical Technology
MAY 19, 2023
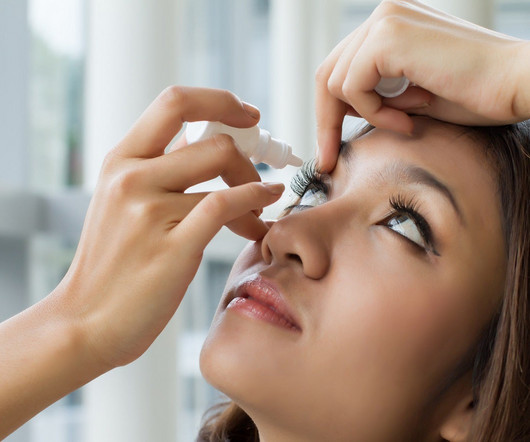
The US Food and Drug Administration (FDA) has granted approval to Bausch + Lomb and Novaliq’s Miebo (perfluorohexyloctane ophthalmic solution) to treat the signs and symptoms of dry eye disease (DED). It is the first and only prescription eye drop to receive FDA approval to treat DED that targets tear evaporation directly.




















Let's personalize your content