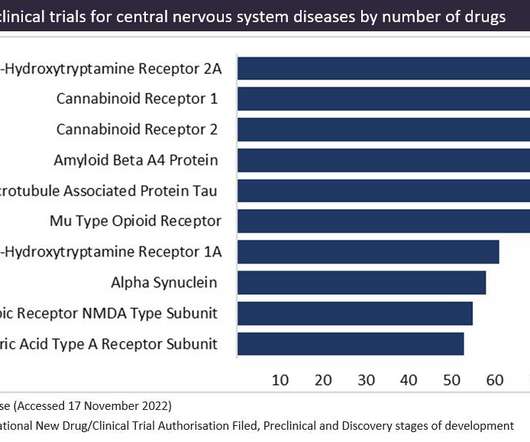
Cannabinoids receptors: popular preclinical target but banned in 137 countries
Pharmaceutical Technology
DECEMBER 5, 2022
Cannabinoid receptors fall into two categories: CB1 and CB2 receptors. Despite the current popularity of cannabinoid receptors, the regulatory landscape is challenging and complex. In the US, the world’s largest pharmaceutical market, most states have legalized cannabinoids for medical use.






















Let's personalize your content