Navigating the Challenges of Biopharma Product Launches: A Blueprint for Success
PM360
FEBRUARY 1, 2024
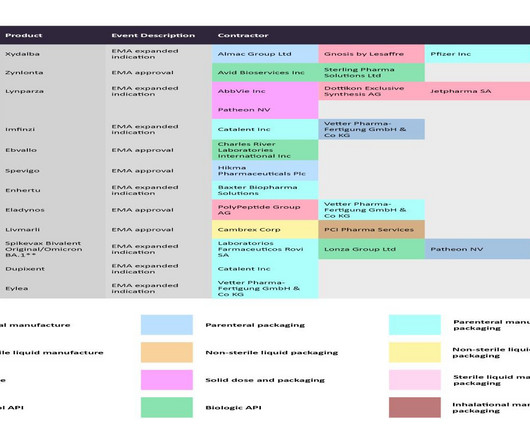
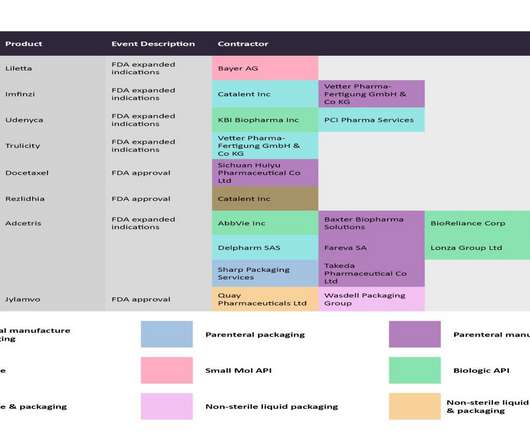
Embarking on the journey from product development to market success in the biopharma industry is no small feat. According to a McKinsey report , a startling 40% of biopharma products fail to meet their sales forecasts within the initial two years, prompting a critical reassessment of strategies for product launches.


















Let's personalize your content