Biopharma Funding Year in Review
Zymewire
JANUARY 26, 2023
Review of 2022 Biopharma funding: How should sales teams at service providers adapt for 2023?

 biopharma-funding-year-in-review
biopharma-funding-year-in-review 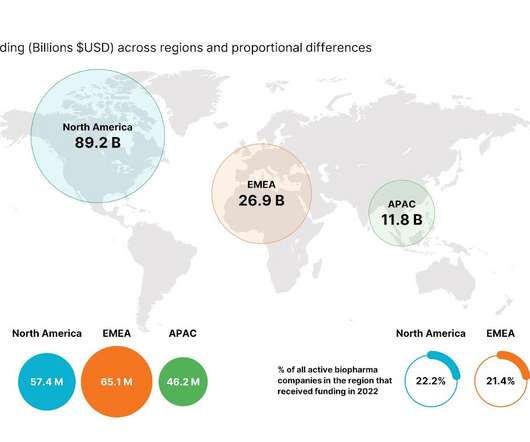
Zymewire
JANUARY 26, 2023
Review of 2022 Biopharma funding: How should sales teams at service providers adapt for 2023?

European Pharmaceutical Review
SEPTEMBER 13, 2023
Following a UK Medicines & Healthcare Products Regulatory Agency (MHRA) inspection, Rentschler Biopharma SE’s UK advanced therapy medicinal products (ATMP) facility has received a current good manufacturing practice (cGMP) Manufacturing Compliance Certificate.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

European Pharmaceutical Review
DECEMBER 30, 2023
The past 12 months has seen significant growth in global mergers and acquisitions (M&A) within the biopharma sector. This activity is expected to continue in 2024, with increasing need for biopharma to access innovation through collaboration and dealmaking to offset looming patent challenges. “In

European Pharmaceutical Review
MAY 2, 2024
The biologics market is expected to continue its fast growth despite the slowdown in funding observed in the past year. The biologics market is expected to continue its fast growth despite the slowdown in funding observed in the past year. What are two current trends you see impacting the biologics field?

European Pharmaceutical Review
JUNE 8, 2023
A new report by the Association of the British Pharmaceutical Industry (ABPI) has identified the UK biopharma industry is increasingly seeking talent with artificial intelligence (AI) and data skills to maintain competitiveness as digital technology continues to drive innovation. This has spiked from 27 in 2019 to 225 in 2022.

European Pharmaceutical Review
SEPTEMBER 26, 2022
“This latest investment is a fantastic opportunity for the Cork site and a recognition of the expertise and commitment of our dedicated employees over the past 20 years. . AbbVie began trading in Ireland in 1974 and has been recognised as one of the Best Large Workplaces in Ireland for several years.

European Pharmaceutical Review
JULY 17, 2023
A new three-year research programme led by researchers at Massachusetts Institute of Technology ( MIT ) is aiming to design the world’s first fully integrated, continuous mRNA manufacturing platform. The $82 million project is funded by the US Food and Drug Administration (FDA) Center for Biologics Evaluation and Research.
Let's personalize your content