Alzheimer’s drug Leqembi set to generate $12.9bn in sales by 2028
Pharmaceutical Technology
MARCH 30, 2023
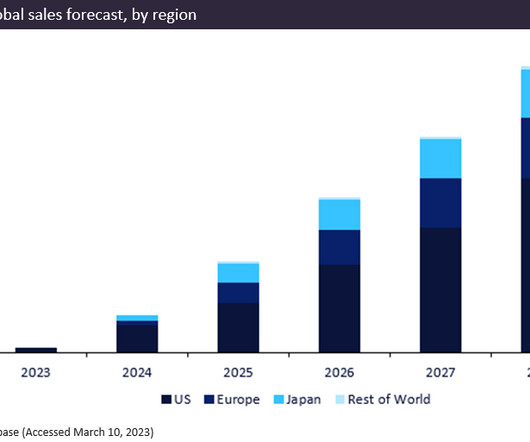
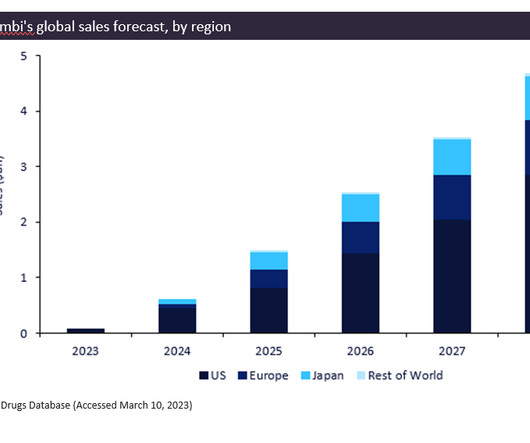
Biogen/Eisai’s newly approved drug, Leqembi (lecanemab), gained FDA approval in January 2023 for the treatment of Alzheimer’s disease. The breakthrough drug is predicted to be a blockbuster, generating total forecast sales of $12.9bn between 2023 and 2028. Leqembi is currently pending approval in the EU and Japan.
























Let's personalize your content