FDA awards full approval to Paxlovid amidst hazy coverage plans
Pharmaceutical Technology
MAY 26, 2023
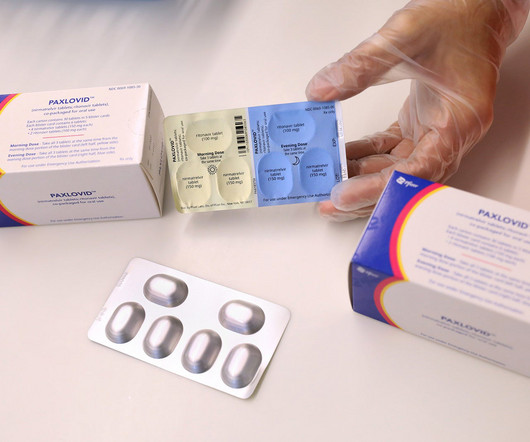
After earning multimillion dollar revenues while being an authorised preferred treatment for Covid-19, the US Food and Drug Administration (FDA) has granted a full approval to Pfizer’s oral antiviral Paxlovid (nirmatrelvir + ritonavir). In response to the approval news, some pointed concerns about long-term safety.
























Let's personalize your content