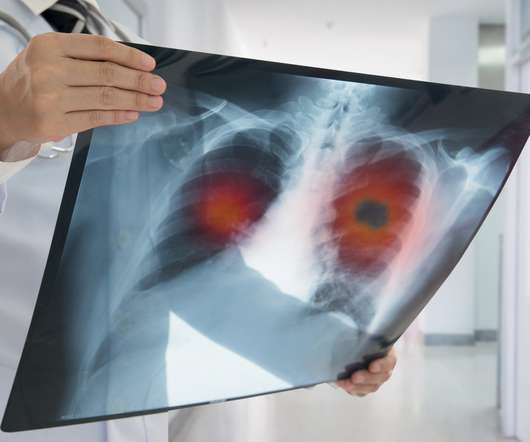
Phase 3 lung cancer fail dents prospects of Ipsen, Servier’s Onivyde
pharmaphorum
AUGUST 3, 2022
Immunotherapies and combination therapies have dramatically improved the prospects for newly-diagnosed SCLC patients, but despite these advances, many rapidly relapse due to the aggressive nature of the disease. million) in sales for the product in the first six months of this year, a rise of 30% at constant exchange rates.















Let's personalize your content