FDA approval for Janssen’s prostate cancer treatment
European Pharmaceutical Review
AUGUST 14, 2023
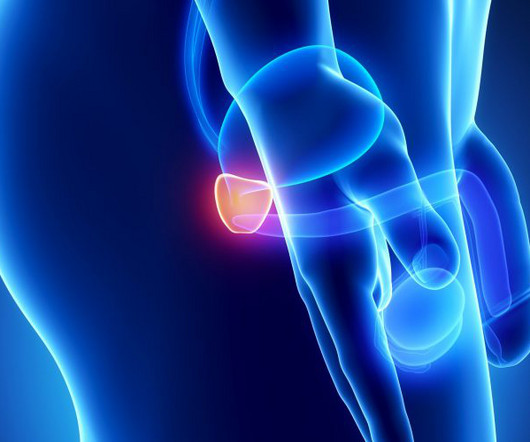
The US Food and Drug Administration (FDA) has approved Janssen’s Akeega (niraparib and abiraterone acetate), for the treatment of adult patients BRCA-positive metastatic castration-resistant prostate cancer (mCRPC). The FDA approval was based on positive results from the multi-centre Phase III MAGNITUDE study.












Let's personalize your content