Cell therapies might revolutionise treatment for multiple sclerosis patients
Pharmaceutical Technology
JANUARY 6, 2023
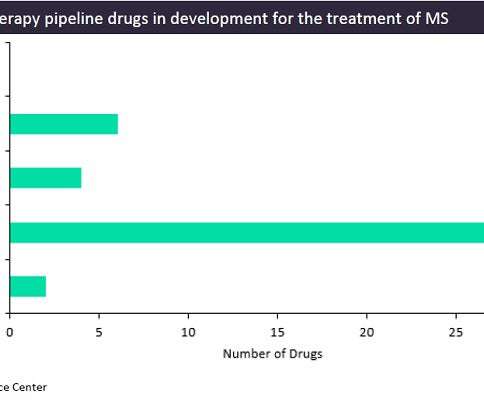
While the treatment options for multiple sclerosis (MS) patients are growing each year with the approval of new agents, all of the currently marketed treatments only slow the disease’s progression and sometimes carry risks of severe side effects, such as liver failure or the development of viral infections.



































Let's personalize your content