Competition is on the horizon for costly narcolepsy treatments
pharmaphorum
DECEMBER 14, 2022
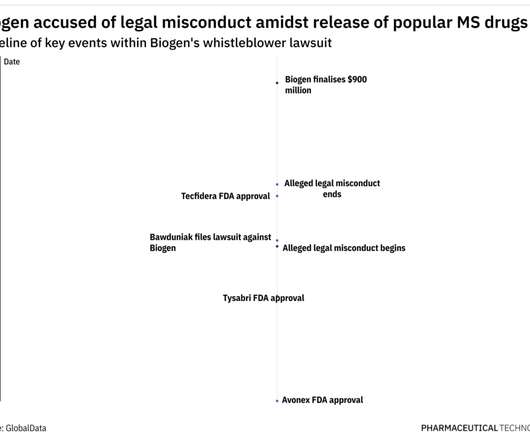
But increased competition is on the horizon. Sodium oxybate — which in the late 1980s was marketed to bodybuilders and then became known as GHB and criminally used as a date rape drug — has been sold under the brand name Xyrem after gaining FDA approval in 2002. Competition starts to heat up. Classic treatment options.













Let's personalize your content