FDA Authorizes Marketing of MISHA™ Knee System for People Suffering from Knee Osteoarthritis
Legacy MEDSearch
APRIL 11, 2023
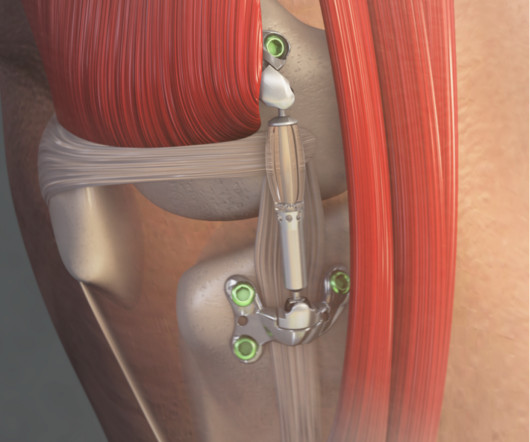
Food and Drug Administration (FDA) granted marketing authorization of the MISHA Knee System, an implantable shock absorber (ISA) for the knee. Chief, Hip and Knee Division of Sports Medicine Institute, Hospital for Special Surgery in New York. and projected to impact 70 million Americans by 2040.

















Let's personalize your content